FDA generic drug approvals rose in 2023 in bid for improved access
Pharmaceutical Technology
FEBRUARY 23, 2024
The FDA Office of Generic Drugs reported a rise in generic drug approvals, as several first-time generics entered the market.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
FEBRUARY 23, 2024
The FDA Office of Generic Drugs reported a rise in generic drug approvals, as several first-time generics entered the market.

Pharmaceutical Technology
OCTOBER 25, 2023
Experts discuss the key trends in quality improvements and API reshoring for the generic drugs market at CpHI Europe.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Patent Watch
OCTOBER 10, 2024
China has emerged as a significant player in the global generic drug active pharmaceutical ingredient (API) market. This article will delve into the role of China in the global generic drug API market, exploring its market share… Source

Drug Patent Watch
SEPTEMBER 26, 2024
The generic drug market is a complex and dynamic environment where multiple factors influence the availability, quality, and pricing of generic drugs. This article will discuss the key strategies and… Source

Drug Patent Watch
DECEMBER 12, 2024
The generic drug market in the United States faces significant challenges, including price volatility, supply chain disruptions, and strategies employed by brand-name manufacturers to delay market entry. These challenges can lead to drug shortages, price spikes, and reduced affordability for patients and payers.
Drug Patent Watch
SEPTEMBER 23, 2024
The generic drug market has experienced significant growth over the past few decades, driven by the expiration of patents on branded drugs and the increasing demand for affordable healthcare solutions. To succeed in this market, generic drug manufacturers must adopt innovative… Source

Drug Patent Watch
OCTOBER 6, 2020
Here is a copy of the talk I gave at the recent Marcusevans 13th Portfolio Management and Pipeline Optimization for Generics. I cover: How to find and evaluate generic entry…. The post Finding and Evaluating Generic Drug Market Entry Opportunities appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
SEPTEMBER 30, 2024
Patent expirations have a significant impact on the pharmaceutical industry, particularly on the generic drug market. When a drug’s patent expires, other manufacturers can produce and market generic versions of the drug, leading to increased competition and lower prices.

Drug Patent Watch
OCTOBER 2, 2023
The FDA conducted a study to identify factors that may predict the likelihood of generic drug marketing applications.

Drug Patent Watch
JULY 25, 2024
The generic drug market has been significantly impacted by the COVID-19 pandemic, with both challenges and opportunities arising from the crisis. As the world continues to recover…

Drug Patent Watch
SEPTEMBER 10, 2024
The generic drug market has experienced significant growth over the past few decades, driven by the passage of the Hatch-Waxman Act in 1984 and subsequent legislation. Today, the market is more competitive than ever, with generic drugs accounting for over 90% of all prescriptions in the United States.

Drug Patent Watch
SEPTEMBER 19, 2024
The generic drug market in the United States is characterized by significant price volatility and shortages, driven by the structure of the market and the incentives for manufacturers. To address these issues, several market-based proposals have been put forth to optimize generic drug cost and availability.

Drug Patent Watch
JUNE 19, 2024
In the ever-evolving pharmaceutical landscape, savvy businesses are constantly on the lookout for untapped markets and lucrative opportunities. One area that has garnered significant attention is the realm of low-competition generic drugs.

Drug Patent Watch
JULY 25, 2024
Developing a competitive edge in generic drug development is crucial for companies to gain a significant market share and dominate the market. Here are some key strategies and insights from industry…

STAT News
MARCH 30, 2023
Supreme Court to review a controversy over so-called skinny labels for medicines, arguing that an appeals court finding threatens the availability of lower-cost generic drugs. For instance, a generic drug could be marketed to treat one type of heart problem, but not another.

BioSpace
JANUARY 14, 2021
Billionaire entrepreneur Mark Cuban, best known as the owner of the Dallas Mavericks and an investor on the ABC business reality series “Shark Tank,” is diving into generic drugs with a new startup, dubbed Mark Cuban Cost Plus Drug Company.

Drug Patent Watch
DECEMBER 11, 2024
The pharmaceutical industry, particularly the generic drug sector, faces significant challenges in adopting sustainable practices. This article outlines key considerations and strategies for developing a sustainable generic drug development strategy. “Generics are known for their cost-effectiveness.

Drug Patent Watch
JANUARY 23, 2025
The Future of Generic Drug Development: Opportunities and Challenges for Emerging Markets As the global healthcare landscape continues to evolve, emerging markets are playing an increasingly important role in shaping the future of generic drug development. Share your thoughts in the comments below!

Drug Patent Watch
DECEMBER 5, 2024
In the ever-evolving landscape of healthcare, generic drugs play a crucial role in providing affordable and accessible medication to millions of people worldwide. But have you ever wondered about the journey these drugs take from conception to your local pharmacy shelf?

Drug Patent Watch
DECEMBER 11, 2024
Generic drugs play a crucial role in providing affordable healthcare options to millions of patients worldwide. One of the key tools that generic drug manufacturers rely on to navigate the complex regulatory environment is the FDA’s Product Specific Guidances (PSGs).

Drug Patent Watch
DECEMBER 10, 2024
India has long been recognized as a significant player in the global pharmaceutical industry, particularly in the production of generic drugs. This article delves into India’s growing importance in generic drug API manufacturing, highlighting the key factors contributing to its success and the challenges it faces.

Drug Patent Watch
JULY 31, 2024
By implementing these strategies, you can can enhance your competitiveness in the market while contributing to increased access to affordable […] Source

Drug Patent Watch
NOVEMBER 18, 2024
The pharmaceutical industry is undergoing a significant shift, with emerging markets offering the next growth opportunity. This growth is driven by several factors, including the increasing prevalence of chronic diseases, cost-effectiveness, and patent expirations of branded drugs.

Pharmaceutical Technology
NOVEMBER 21, 2022
Japan is currently the fourth largest market in the world. Based on GlobalData estimates, the Japanese pharmaceutical market generated JPY9.392 trillion ($67.32 Other contributing factors include the decreasing number of post-market studies and difficulties in primary research and manufacturing.

Drug Patent Watch
JANUARY 21, 2025
Navigating the Complex World of Generic Drug Development: Risk Management Strategies to Know As a generic drug developer, you're no stranger to the challenges of bringing affordable medications to market. One of the biggest risks generic drug developers face is patent infringement.
Outsourcing Pharma
OCTOBER 12, 2020
Technology impacting generic drugs include artificial intelligence, telemedicine, fraud-preventing digital solutions and more, according to a report.

Drug Patent Watch
DECEMBER 30, 2024
The regulatory environment in Japan for generic drug development is complex and has undergone significant changes in recent years. Regulatory Authority: Pharmaceuticals and Medical Devices Agency (PMDA) The PMDA is the primary regulatory authority responsible for overseeing the drug approval process in Japan.

NY Times
SEPTEMBER 18, 2021
Competition for market share at rock-bottom prices has led to shortages, price-spikes, allegations of price-fixing, and substandard and even dangerous practices.
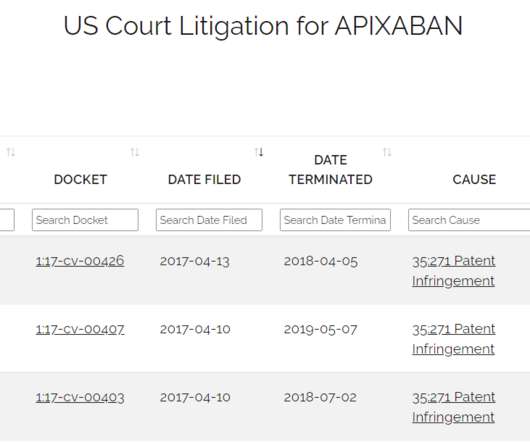
Drug Patent Watch
AUGUST 12, 2020
Just because a drug has received FDA approval does not mean that it is available in the marketplace. The post Generic Drugs Approved but not Launched – How to Tell When Generic Drugs Will hit the Market appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
JANUARY 14, 2025
The Generic Drug Revolution: Setting the Stage Before we dive into the nitty-gritty, let’s take a moment to appreciate the impact of generic drugs. Why Generic Drugs Matter Generic drugs are the Robin Hoods of the pharmaceutical world, stealing from the rich (brand-name drugs) and giving to the poor (our wallets).
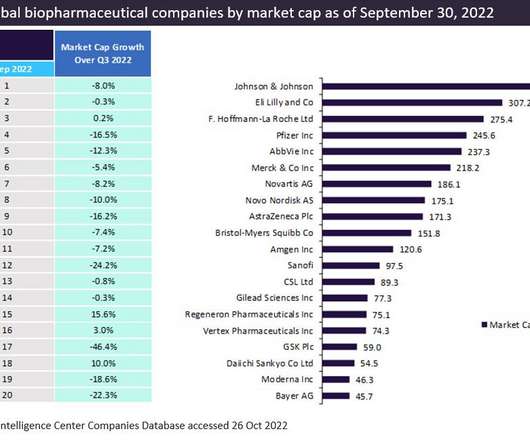
Pharmaceutical Technology
DECEMBER 20, 2022
The top 20 global biopharmaceutical companies exhibited a downward trend in aggregate market capitalisation by 9.1% This downturn in market cap was attributed to a decline in the demand for Covid-19 vaccines and therapies. Bayer recorded a negative market capitalization growth of 22.3% trillion in Q2 2022 to $3.14

STAT News
MARCH 30, 2023
What happened to the market for monkeys? Are some drugs too cheap? We also discuss what leads to generic drug shortages, whether every major pharmaceutical firm needs a weight-loss drug, and what it means when drug company cancels a conference appearance. And why are biotech stocks still in the tank?

Drug Patent Watch
DECEMBER 10, 2024
Generic drug development is a complex process that requires a deep understanding of regulatory requirements and guidelines. Regulatory expertise plays a crucial role in ensuring that generic drugs meet the necessary standards for quality, safety, and efficacy. EU, and other regions.

Pharmaceutical Technology
SEPTEMBER 15, 2022
Various factors have contributed to the need and growth of API chemical suppliers such as rising healthcare expenditure, increasing disposable incomes, growing geriatric population, increasing incidence of chronic diseases, patent expiration of blockbuster drugs, increased consumption of generic drugs, and intervention of the new generation APIs.
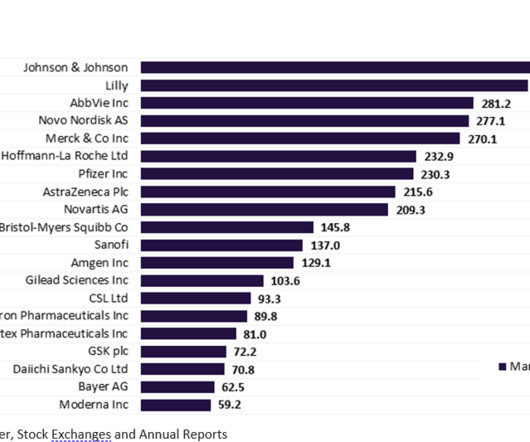
Pharmaceutical Technology
MAY 16, 2023
More than half of the top 20 global biopharmaceutical companies saw a fall in market capitalisation over Q1 2023. decline in total aggregate market capitalisation from $3.61 Bayer reported the highest market capitalisation growth of 23.1% Sanofi and Regeneron’s market capitalisation grew by 12.4% This resulted in a 3.4%

Drug Patent Watch
JANUARY 29, 2025
As we navigate the complex world of healthcare, it's easy to overlook the intricacies of generic drug production. We often assume that generic medications are cheaper because they're, well, generic. Generic drug production involves replicating a brand-name medication's active ingredient, formulation, and dosage.

Pharmaceutical Technology
NOVEMBER 21, 2022
Japan is currently the fourth largest market in the world. Based on GlobalData estimates, the Japanese pharmaceutical market generated JPY9.392 trillion ($67.32 Other contributing factors include the decreasing number of post-market studies and difficulties in primary research and manufacturing.

Pharmaceutical Technology
JULY 25, 2022
Despite this, specialty generics are expected to be the domain of a handful of companies with the necessary manufacturing capabilities and legal backing needed for entering the market. The only way forward for generics producers. More specifically, Frank says drug-device combinations share some of these characteristics.

Pharmaceutical Technology
OCTOBER 5, 2008
In addition, one very notable deal with India hints that Asia’s emerging market is also having a growing influence on what is happening in Japan. ’ Generic drug renaissance Shionogi’s acquisition of Sciele was partly due to the US business experience of Shionogi’s chief executive, Isao Teshirogi.

BioTech 365
JUNE 25, 2021
GCC Generic Drug Market Trends, Share, Size, Opportunity and Forecasts 2021-2026 – ResearchAndMarkets.com GCC Generic Drug Market Trends, Share, Size, Opportunity and Forecasts 2021-2026 – ResearchAndMarkets.com DUBLIN–(BUSINESS WIRE)–The “GCC Generic Drug Market: Industry Trends, Share, Size, Growth, Opportunity and Forecast (..)

Drug Patent Watch
JANUARY 22, 2025
The Unseen Journey of Generic Drugs: A Look into the Regulatory Pathway Have you ever wondered how generic drugs make it to the market? As a healthcare professional or a pharmaceutical enthusiast, understanding the pathway to generic drug approval can be fascinating.
Drug Patent Watch
JUNE 18, 2024
Branded generics are generic drugs that are marketed under a brand name by the manufacturer. Branded generics can be an attractive option for both consumers and pharmaceutical companies, offering cost savings while leveraging brand recognition. […] Source

World of DTC Marketing
APRIL 12, 2021
The goal of getting a drug to market as fast as possible so they can recoup drug development costs has the potential for mistakes that could cost lives. Coupled with all this is the continued outsourcing of raw materials and other steps in drug development. Patients should be scared.

BioTech 365
MAY 28, 2021
Global Generic Drugs Market Report 2021: The New Generics Era – The Patent Cliff, Types of Generic Drugs, Simple Generics, Super Generics, Biosimilars, ANDA Approvals – ResearchAndMarkets.com Global Generic Drugs Market Report 2021: The New Generics Era – The Patent … Continue reading →
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content