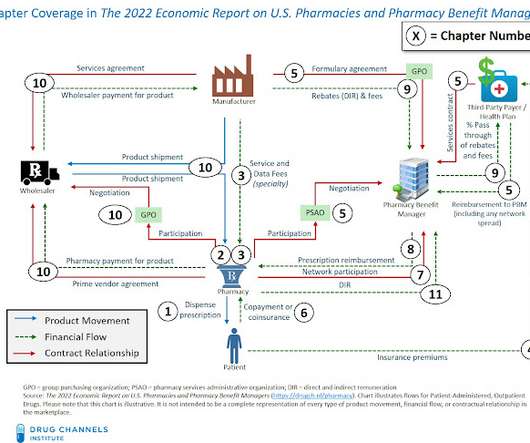
NOW AVAILABLE: The 2022 Economic Report on U.S. Pharmacies and Pharmacy Benefit Managers
Drug Channels
MARCH 15, 2022
Pharmacies and Pharmacy Benefit Managers , available for purchase and immediate download. Click here to download a free report overview (including key industry trends, the Table of Contents, and a List of Exhibits) New Drug Channels Institute Study Examines Consolidation and Vertical Integration of U.S.

























Let's personalize your content