Novartis to consider sale or separation of Sandoz business
Bio Pharma Dive
OCTOBER 26, 2021
The generic drug unit has struggled in recent years, while Novartis has moved to focus more narrowly on higher-margin branded medicines.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
OCTOBER 26, 2021
The generic drug unit has struggled in recent years, while Novartis has moved to focus more narrowly on higher-margin branded medicines.
Pharmaceutical Technology
MAY 3, 2023
Since 2015, the US Food and Drug Administration (FDA) has approved more than 450 “first generics” or the first generic equivalent for a branded drug. These medicines comprise about 10% of all generics approved each year.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmacy Checkers
DECEMBER 5, 2019
A new Chinese law (in effect as of December 1, 2019) makes third-party online platform medicine sales legal, appropriately ends a draconian definition of counterfeit drugs, and effectively decriminalizes personal drug importation, but with a lot of gray! Buying Drugs Online from Retail Platforms is Legal.

Camargo
NOVEMBER 11, 2020
Presumably, Antares’ long-established 90-person urology sales force can achieve pull-thru of this drug product. Labeling regulations prohibit reminder ads for products with boxed warnings, which the FDA requires when a drug product has very serious risks. In 2019, Foundation Medicine and Bayer Healthcare Pharmaceuticals, Inc.

pharmaphorum
NOVEMBER 8, 2021
The largest left-leaning party in Switzerland said that national ownership of Sandoz would democratise the production and development of new medicines, maintain a stable supply of affordable drugs, and allow less profitable R&D to be carried out with state support.

Pharmaceutical Technology
NOVEMBER 21, 2022
Based on GlobalData estimates, the Japanese pharmaceutical market generated JPY9.392 trillion ($67.32 billion) in total sales in 2021 and is expected to grow 1.1% Among the 37 member companies, 17 of them are overseas companies and 20 of them are Japanese companies with a 10%+ overseas pharma sales ratio.

Pharmaceutical Technology
OCTOBER 5, 2008
’ Generic drug renaissance Shionogi’s acquisition of Sciele was partly due to the US business experience of Shionogi’s chief executive, Isao Teshirogi. ” The move, which came in June, also gives Japanese drugs maker Daiichi Sankyo a foothold in the Indian market through Ranbaxy’s location. .”

pharmaphorum
AUGUST 20, 2020
Shares in Teva were down sharply this week after the US Department of Justice filed a complaint that the company paid illegal kickbacks to patient groups to boost sales of its multiple sclerosis drug Copaxone (glatiramer). The allegation is that Teva’s subsidiaries Teva Pharmaceuticals USA Inc. and Teva Neuroscience Inc.

Pharmaceutical Technology
NOVEMBER 21, 2022
Based on GlobalData estimates, the Japanese pharmaceutical market generated JPY9.392 trillion ($67.32 billion) in total sales in 2021 and is expected to grow 1.1% Among the 37 member companies, 17 of them are overseas companies and 20 of them are Japanese companies with a 10%+ overseas pharma sales ratio.

XTalks
JANUARY 20, 2025
While the deal required regulatory divestitures, including the sale of Otezla (apremilast) to Amgen for $13.4 This strategic move transformed Actavis into one of the largest pharma companies globally, with a strong presence across branded, generic and over-the-counter (OTC) medicines.

pharmaphorum
MARCH 5, 2021
Copaxone (glatiramer) was once a mainstay of Teva’s portfolio with blockbuster sales that the Israel-based pharma fought hard to protect, including releasing a long-acting version to counter cheaper copycat drugs. In the end it wasn’t enough and sales last year were $213 million, a fraction of the $4.3

pharmaphorum
JANUARY 25, 2022
.” The online pharmacy launch comes a few weeks after Cuban set up a pharmacy benefit manager (PBM) operation – the Mark Cuban Cost Plus Drug Company (MCCPDC) – promising to cut out the middleman in the medicines supply chain so it can eliminate markups on generic drugs and pass savings on to its customers.

Pharmaceutical Technology
JULY 10, 2008
As the blockbuster drugs of the 90s that earned the industry billions reach their patent shelf lives, pharmaceutical companies require new medicines to sustain an estimated $157bn worth of sales. Those 38 products generated just $10bn of the $316bn industry’s entire portfolio of medicines.

pharmaphorum
NOVEMBER 26, 2020
For Pawlu, COVID-19 has highlighted how we transform the way the industry works – from manufacturing and supply chains, to sales and detailing – in a way that ensures everyone can access the high quality, affordable medicines they need to live happier, healthier lives. About the interviewee. About the author.

pharmaphorum
JULY 29, 2021
In a statement, Advanz said that it “utterly disagrees” with the CMA’s decision, saying it invested significantly to keep the product on the market to the specifications required by the Medicines and Healthcare products Regulatory Agency (MHRA). ” Advanz itself was directly fined £40.9 million and £51.9
pharmaphorum
MARCH 16, 2022
Viatris’ generic of Symbicort (budesonide and formoterol fumarate dihydrate) – developed by Viatris in collaboration with 3M spin-off company Kindeva Drug Delivery and called Breyna – has been cleared for the same indications as the brand, namely maintenance treatment for asthma and chronic obstructive pulmonary disease (COPD).

Pharma in Brief
JUNE 21, 2020
On June 19, 2020, the Patented Medicine Prices Review Board ( PMPRB ) launched its consultation on revised Draft Guidelines to implement the amended Patented Medicines Regulations. Amendments to the Patented Medicines Regulations are scheduled to come into force on January 1, 2021. New patented medicines. Background.

pharmaphorum
JUNE 3, 2021
The US regulator approved Lybalvi (olanzapine/samidorphan) for both indications sought – schizophrenia and bipolar I disorder – with data on its label that includes a claim of less weight gain with olanzapine on its own, a big problem with the widely-used generic drug that affects compliance with treatment. In its favour?

Druggist
SEPTEMBER 30, 2020
Buscopan vs Mebeverine over the counter availability and age restrictions The table below summarises over the counter availability of both drugs and age restrictions associated with each brand. Pharmacy-only medicines can only be purchased from the pharmacy counters, including online chemist. Can you buy Buscopan over the counter?

The Pharma Data
DECEMBER 4, 2021
is a topical prescription medicine used to treat acne vulgaris. The launch of our first-to-market authorized generic version of Epiduo® Forte Gel in the U.S. provides patients with another important treatment option,” said Christine Baeder, SVP, Chief Operating Officer US Generics, Teva USA. IMPORTANT SAFETY INFORMATION.

FDA Law Blog
JUNE 16, 2023
Kirschenbaum — On May 24, Minnesota enacted the Commerce and Consumer Protection Omnibus Bill, Senate File 2744 ( SF 2744 ), which significantly expands the state’s existing drug pricing activities with serious implications for all drug manufacturers, and particularly generic drug manufacturers.

Pharmacy Checkers
DECEMBER 26, 2019
Still, the government’s most recent survey data, published in 2015, showed that about four million Americans import medicine each year because of cost. [vi]. Federal law provides extensive flexibility for Americans to personally import more affordable medicine as long as it doesn’t present an unreasonable risk to the patient.

The Pharma Data
JANUARY 27, 2021
Further to XPhyto’s press release dated January 18, 2021, the Company’s core milestones for this year include the commercialization of infectious disease diagnostics, the clinical validation of transdermal and sublingual drug formulations and continued investment in psychedelic medicine. About XPhyto Therapeutics Corp.

The Pharma Data
JANUARY 17, 2021
The Company will continue to leverage its scientific expertise and operations in Europe and North America for product development and optimization while it plans to add significant commercial experience in the fields of manufacturing, distribution, marketing and sales. Diagnostics.

The Pharma Data
OCTOBER 14, 2020
“With the Court’s approval of our First Day motions, we can ensure uninterrupted patient access to our medicines while we move through this process. Its Specialty Generics reportable segment includes specialty generic drugs and active pharmaceutical ingredients.
XTalks
FEBRUARY 5, 2024
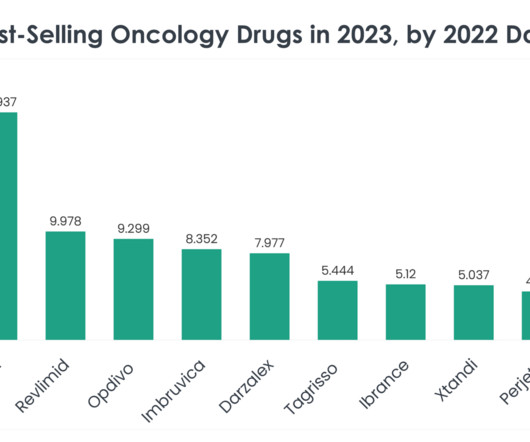
In the dynamic landscape of oncology therapeutics, understanding the trends and performances of leading drugs is crucial for stakeholders across the healthcare and life sciences sectors. The top 40 best-selling oncology drugs in 2023, informed by 2022 sales statistics, mark significant milestones in cancer treatment and research.
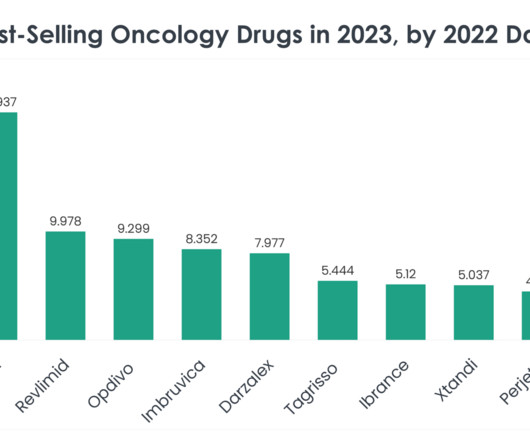
XTalks
FEBRUARY 5, 2024
In the dynamic landscape of oncology therapeutics, understanding the trends and performances of leading drugs is crucial for stakeholders across the healthcare and life sciences sectors. The top 40 best-selling oncology drugs in 2023, informed by 2022 sales statistics, mark significant milestones in cancer treatment and research.
The Pharma Data
MARCH 9, 2021
“This first-to-market generic version of AZOPT ® (brinzolamide ophthalmic suspension) 1% in the U.S. provides patients with another important treatment option,” said Christine Baeder, SVP, Chief Operating Officer US Generics, Teva USA. Currently, one in ten generic prescriptions dispensed in the U.S.
pharmaphorum
MARCH 3, 2022
Non-profit drugmaker Civica Rx has said it will launch biosimilars of three big-selling insulin products in the US by 2024 to help diabetic patients struggling with the cost of the drugs. Cheaper options are meanwhile becoming available. The post Civica plans shake-up of insulin sector with low-cost biosimilars appeared first on.

The Pharma Data
MARCH 7, 2022
(NYSE and TASE: TEVA), announced today the launch of a first generic version of Revlimid® 1 (lenalidomide capsules), in 5mg, 10mg, 15mg, and 25mg strengths, in the United States. Please see the below “What are lenalidomide capsules?” “The launch of our first generic version of Revlimid® in the U.S. Revlimid® had annual sales of $2.3

Pharma in Brief
JANUARY 9, 2022
These policy initiatives included reforms to the Patented Medicine Prices Review Board ( PMPRB ), the Patented Medicines (Notice of Compliance Regulations) ( PM(NOC) Regulations ), the Patent Rules , and other regulatory matters at Health Canada. The courts also had a busy year in 2021. Major Policy Initiatives. (a) a) COVID-19.

pharmaphorum
MARCH 23, 2022
All three have announced price cuts in the last couple of years, but critics say these do not go far enough to help people with diabetes who are struggling to afford their medicines. Assuming the bill is later passed by the Senate and signed into law by President Biden, the insulin cap would take effect beginning in 2023.
Druggist
MARCH 17, 2021
Lymecycline is usually prescribed as a generic drug – lymecycline 408mg capsules or occasionally as a branded medication called Tetralysal 300 mg capsules. Both drugs are the same medicines. How does lymecycline work for acne? Freederm gel is available on Amazon.co.uk (link below). £6.39. −£1.89.

XTalks
AUGUST 2, 2023
With each passing year, pharmaceutical companies around the globe strive to deliver cutting-edge medicines, therapies and vaccines that impact the lives of millions. In this in-depth article, we embark on a captivating journey to uncover the top 30 pharma and biotech companies that have been instrumental in shaping the future of medicine.

XTalks
APRIL 10, 2025
In 2024, the US pharmaceutical market reached an estimated $639billion in sales , of which roughly $213billion or onethird was met by imports. In 2023, Ireland, for instance, earned over $51billion from US drug exports, followed by Germany at $20billion, India at $11billion and China at $7.8billion the latter which now faces 125% tariffs.

The Pharma Data
MAY 4, 2021
Internal Medicine. Following the completion of the spin-off of the Upjohn Business (4) in the fourth quarter of 2020, Pfizer now operates as a focused innovative biopharmaceutical company engaged in the discovery, development, manufacturing, marketing, sales and distribution of biopharmaceutical products worldwide. First-Quarter. .

Pharmacy Checkers
MARCH 11, 2020
tolerates this magnitude of cost-related medication non-adherence, in which people die because of drug prices, should shock our national conscience. [2]. 381), if their imported prescription orders are refused so that they are released to those patients who provide ample evidence to the FDA that their medicine is not an unreasonable risk.
Pharmacy Checkers
MAY 1, 2020
I’m a big fan of testing prescription drugs to see if they have the right stuff (to put it eloquently). Here’s why: lots of pharma-funded groups and some people at the FDA say don’t buy medicine online from foreign countries because it’s allegedly unsafe. Bayer’s “pat-on-the back” press release did not mention where Resochin is made.

FDA Law Blog
SEPTEMBER 13, 2021
Readers following drug pricing activity will recognize several strategies already associated with Senate Democrats (e.g., Medicare negotiation authority; encourage biosimilars and generic drug utilization). The HHS plan’s proposals are defined in general terms, without estimates of taxpayer savings or timelines.

XTalks
SEPTEMBER 14, 2020
If retail sales were the sole indicator that the US economy is recovering, the country would have something to cheer about. According to the Commerce Department, retail sales rose 7.5 Retail sales in the UK have bounced back to relatively high levels, even though initially, sales were extremely anemic, with only a 0.9
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content