Genetically modified herpes combats advanced cancers
Pharma Times
SEPTEMBER 21, 2022
A new genetically engineered virus has delivered a one-two punch in initial phase 1 trial
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharma Times
SEPTEMBER 21, 2022
A new genetically engineered virus has delivered a one-two punch in initial phase 1 trial
AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 7, 2023
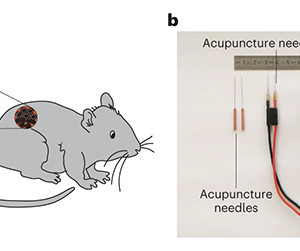
New research from ETH Zürich in Switzerland could see future wearable devices (with perhaps a few implants and a touch of genetic engineering) boost our health directly. Fitness trackers help you stay healthy by keeping count of your steps and monitoring your heart rate, driving you on to hit those cardio goals.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JUNE 10, 2023
CIGB-128 is under clinical development by Center for Genetic Engineering and Biotechnology and currently in Phase I for Brain Tumor. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It is a combination of interferon alfa-2b with interferon gamma.

Pharmaceutical Technology
JUNE 10, 2023
CIGB-128 is under clinical development by Center for Genetic Engineering and Biotechnology and currently in Phase I for Brain Tumor. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It is a combination of interferon alfa-2b with interferon gamma.
Pharmaceutical Technology
MAY 10, 2023
SYNB1934 consumes Phe in the gastrointestinal (GI) tract by leveraging genetic engineering of the drug or drug-carrying capsule, probiotic escherichia coli (E coli) Nissle. This designation also comes at a pivotal time as we prepare to initiate our Phase 3 trial for PKU – Synpheny-3 – in the first half of 2023.”
Pharmaceutical Technology
SEPTEMBER 5, 2022
In March last year, CanSinoBIO obtained approval for the clinical trial application to analyse Convidecia Air. Administered as a single dose, the genetically engineered vaccine has the replication-defective adenovirus type 5 vector that expresses the spike S protein of the SARS-CoV-2 virus.

Advarra
JUNE 13, 2024
Recombinant DNA technologies and genetically modified biological agents are being adapted for a wide scope of therapeutic applications, and their use is becoming increasingly common in clinical trials. How can I protect myself from exposure? What should I do if I’m exposed?
BioSpace
APRIL 25, 2024
The genetic engineering startup, recently honored by BioSpace readers for its work environment, is downsizing as it seeks to launch its first clinical trials.
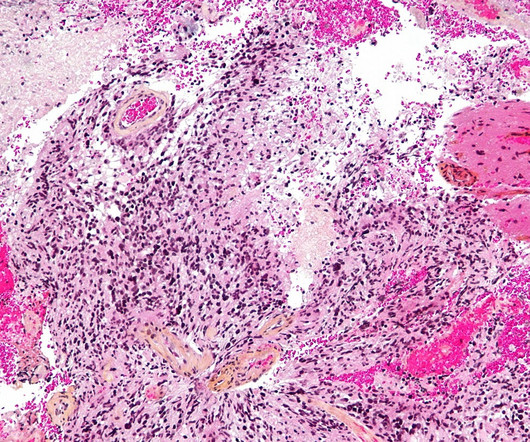
Pharmaceutical Technology
APRIL 26, 2023
This marks the first-ever designation for genetically modified gamma-delta T cell therapies. INB-400, an autologous, genetically engineered gamma-delta T cell therapy, is the company’s DeltEx chemotherapy-resistant autologous and allogeneic drug-resistant immunotherapy (DRI) technology.
STAT News
JULY 11, 2022
Then it was almost instantly derailed by the death of an 18-year-old clinical trial volunteer named Jesse Gelsinger after he received a genetically engineered virus that had been developed to treat his rare liver condition.

BioSpace
DECEMBER 6, 2021
Proof of concept for the Genetically Engineered Microbial Medicines (GEMM) platform came in a recent first-in-human, Phase I trial.

Pharmaceutical Technology
JUNE 29, 2022
In September 2021, GlobalData figures revealed there to be 1,320 industry-sponsored regenerative medicine and advanced therapy trials ongoing worldwide. Novozymes has a long legacy of enzyme discovery and the ability to genetically engineer these speciality enzymes to be superior. And it’s within the family – Novozymes!”.

pharmaphorum
SEPTEMBER 11, 2022
It is the first time that a cell therapy for solid tumours has been tested in a phase 3 trial, and the first time that the approach has been directly compared with standard second-line immunotherapy in melanoma. ” The post ESMO: TIL therapy improves on Yervoy in melanoma trial appeared first on.

Pharmaceutical Technology
MARCH 2, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. The therapeutic candidate comprises allogeneic NK cells genetically engineered to express chimeric antigen receptors (CAR-NK) targeting cells CD33.

pharmaphorum
NOVEMBER 1, 2021
Delytact (teserpaturev) is a genetically engineered oncolytic herpes simplex virus type 1 (HSV-1) that was approved for marketing in Japan earlier this year, and received pricing approval in August at 1.43 million yen (around $12,500) per dose, according to a Pharma Japan report.
Advarra
MARCH 31, 2023
The use of engineered genetic materials in clinical trials is rapidly expanding, with potential applications for genetic vaccines, gene-modified cellular therapies, and gene therapies. A key part of the IBC’s evaluation is assessing the risks posed by the engineered genetic materials.

Pharmaceutical Technology
AUGUST 17, 2022
These capabilities comprise cell and exosome genetic engineering for expressing therapeutic targets. In order to offer complete process development, quality control and regulatory services, cGMP manufacturing for pre-clinical and Phase I/II clinical trials, AGC will use its worldwide network.
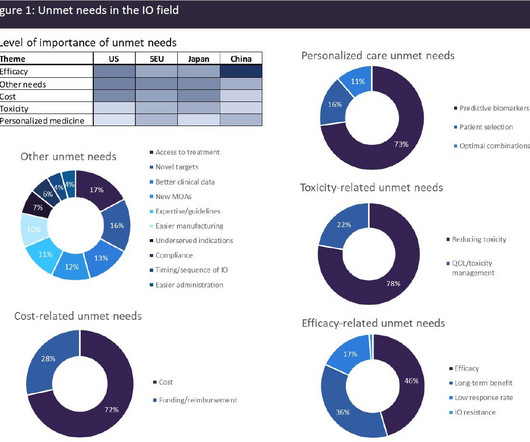
Pharmaceutical Technology
MAY 2, 2023
Chimeric antigen receptor T-cell (CAR-T) therapies are the only genetically modified cell therapies to have received regulatory approval, and they are currently utilised in relapsed/refractory settings. There is currently a rich and diverse IO pipeline that aims to address these unmet needs, with 703 IO products currently in clinical trials.

Velocity Clinical Research
OCTOBER 3, 2024
.” All Velocity sites are fully prepared to support cell and gene therapy (CGT) clinical trials. Read the full press release: [link] The post Nick Spittal Comments on Velocity’s Membership With Advarra’s Gene Therapy Ready Site Network appeared first on Velocity Clinical Research.

Pharmaceutical Technology
FEBRUARY 15, 2023
Innovation S-curve for the pharmaceutical industry Gene splicing using nucleases is a key innovation area in the pharmaceutical industry Nucleases play a fundamental role in the field of recombinant DNA technology, or genetic engineering. Gene splicing using nucleases is used to design gene therapeutics for various genetic disorders.
XTalks
NOVEMBER 23, 2020
Severe cases of the infection did not occur among trial participants, nor were any hospitalizations reported. The global trials are assessing the safety and efficacy of the vaccine in individuals aged 18 years or over from diverse racial and geographic groups who are healthy or have stable underlying medical conditions.

pharmaphorum
JANUARY 26, 2023
Yescarta – a CD19-directed genetically modified autologous T-cell immunotherapy investigated in the ZUMA-1 trial – is the first chimeric antigen receptor (CAR) T-cell therapy recommended for routine use on the NHS in England , meaning that for the first time eligible patients will be able to access CAR-T cell therapy in the long-term.
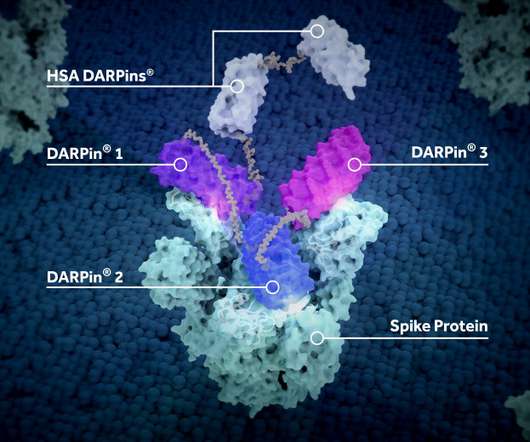
pharmaphorum
JANUARY 10, 2022
The opt-in has been prompted by the results of a phase 2 trial of ensovibep (MPO420), which showed that a single intravenous dose of the drug was able to not only reduce viral load in non-hospitalised COVID-19 patients over eight days, but also cut the risk of hospitalisation or death by 78% versus placebo.

pharmaphorum
NOVEMBER 6, 2020
Novartis’ Ilaris (canakinumab) has failed to produce results in a phase 3 trial, which tested whether it could improve COVID-19 patients’ survival chances without need for mechanical ventilation. The key secondary endpoint was to reduce the COVID-19-related death rate during the four week period after trial treatment.
XTalks
JULY 31, 2020
The study developed and researched microbe-controlled and genetically-engineered mice to understand the healing response in the intestine when receiving specific signals from bacteria. If we can influence these interactions, we may be able to control many diseases that are impacted by our microbiome or diet,” she says.
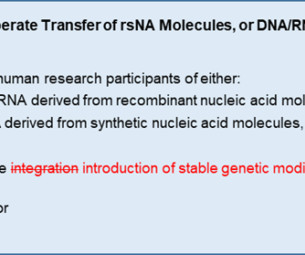
WCG Clinical
NOVEMBER 17, 2023
Since then, however, certain genetic engineering technologies (e.g., For example, two trials testing genetically identical genome-edited cell therapies – one engineered with a viral vector, and one without – can differ in their IBC review requirements because of how the cells were modified rather than what they have become.

Advarra
AUGUST 8, 2023
Gene therapy involves the transfer of engineered genetic materials to human research subjects. These studies were previously considered to be highly experimental and limited to early phase trials at a handful of highly specialized academic medical centers.
Pharmaceutical Technology
APRIL 5, 2023
And my estimate is that between 25,000 and 30,000 patients have been treated with those therapies globally, which does not include patients treated in clinical trials. We have the emergence of this new pillar of medicine, which is out of the lab and beyond clinical trials now. But there is that significant challenge.
Advarra
JUNE 13, 2023
Research in gene therapies and genetically engineered drugs and vaccines are growing exponentially, and will only continue to become more popular. Additionally, in some cases, gene therapy treatments make permanent changes to a human’s genetic profile, which is different than typical drug treatments.

pharmaphorum
JULY 27, 2021
AstraZeneca has formed a partnership with Regeneron to investigate the potential of treating obesity using drugs directed at GPR75, a protective gene identified by scientists at the Regeneron Genetics Centre.

pharmaphorum
FEBRUARY 18, 2021
Researchers from the University of Texas Medical Branch based their findings on lab tests using SARS-CoV-2 coronaviruses that were genetically engineered to have the same mutations as those in the strain that is causing scientists so much concern.

Advarra
JANUARY 11, 2024
Adoptive T Cell therapies, therapeutic antibodies, and immunomodulatory proteins represent just some of the potentially beneficial treatment strategies for successful mRNA cancer trials. Only after these trials are successful can sponsors seek market approval for mRNA-based products.

NY Times
FEBRUARY 22, 2021
Two patients in a gene therapy study developed cancer years after treatment. It is not clear whether the therapy was responsible.

The Pharma Data
NOVEMBER 30, 2020
CoV2373 is a stable, prefusion protein antigen derived from the genetic sequence of the SARS-CoV-2 coronavirus spike (S) protein and adjuvanted with Novavax’ proprietary Matrix?M. pivotal Phase 3 trial update. Novavax completed enrollment of 15,000 participants in a pivotal Phase 3 clinical trial being conducted in the U.K.

The Pharma Data
JUNE 25, 2021
efficacy overall, and met the primary endpoint in its PREVENT-19 pivotal Phase 3 trial. “These data show consistent, high levels of efficacy and reaffirm the ability of the vaccine to prevent COVID-19 amid ongoing genetic evolution of the virus. All cases observed in the vaccine group were mild as defined by the trial protocol.

The Pharma Data
NOVEMBER 9, 2020
Novavax expects to begin its pivotal Phase 3 clinical trial in the United States and Mexico by the end of November. Data from the event-driven trial could support global authorization and approval, including in the U.S. and globally.”. About NVX-CoV2373. and Australia. billion from the U.S. government.
Bioengineer
JULY 19, 2021
The researchers at the CNIO created two genetically engineered mouse models that lacked KRAS4B and expressed the KRAS4A variant only, both with and without the G12V mutation (KRAS4AG12V). Our results suggest that for therapies to be effective, the two KRAS isoforms should be targeted.” ” KRAS genes in embryonic development.

XTalks
MARCH 28, 2024
For CRC, treatment options that are targeting genetic mutations such as BRAF , KRAS and HER2 have been developed. Early clinical trials have shown promising results and this therapy could become a valuable addition towards CRC treatment in the future. Yang Liu and CFO Abid Ansari – Xtalks Life Science Podcast Ep.

The Pharma Data
DECEMBER 16, 2020
NVX-CoV2373 is a protein-based vaccine candidate engineered from the genetic sequence of SARS-CoV-2, the virus that causes COVID-19 disease. NVX-CoV2373 is being evaluated in an ongoing Phase 3 trial in the U.K. Additional terms of the agreement were not disclosed. About NVX-CoV2373. and Australia. About Matrix-M.

The Pharma Data
JANUARY 10, 2021
The new year began with a fairly low level of clinical trial news. Arcturus Therapeutics got the FDA go-ahead for its Phase II trial of its COVID-19 vaccine candidate ARCT-021. The trial will enroll 600 participants, with 450 receiving ARCT-021 and 150 receiving placebo. Here’s a look. COVID-19-Related. Non-COVID-19-Related.

Bioengineer
JULY 21, 2021
Its biotechnology trade magazine, GEN (Genetic Engineering & Biotechnology News), was the first in its field and is today the industry’s most widely read publication worldwide. Mary Ann Liebert, Inc., A complete list of the firm’s 90 journals, books, and newsmagazines is available on the Mary Ann Liebert, Inc.,

Bioengineer
JULY 15, 2021
Its biotechnology trade magazine, GEN (Genetic Engineering & Biotechnology News), was the first in its field and is today the industry’s most widely read publication worldwide. Mary Ann Liebert, Inc., publishers (https:/ / www. liebertpub. com/ ) website.

Advarra
AUGUST 10, 2023
How Can Research Professionals Mitigate Risks with Engineered Genetic Materials? IBCs are charged with protecting study personnel, the community, and the environment from exposure to engineered genetic material. Gene therapy products may pose unknown risks, and Dr. Marks said there is much to learn about the field. “We

The Pharma Data
MARCH 29, 2022
In a preprint, the researchers at Cornell University led by Associate Professor Hector Aguilar-Carreno showed that genetically engineered mice infected with the virus causing COVID-19 and given a daily dose of the compound in a nasal spray for four days.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content