Scribe and Sanofi expand genetic therapy development deal
Pharmaceutical Technology
JULY 18, 2023
Scribe Therapeutics and Sanofi have expanded partnership to progress the development of in vivo genetic therapies to treat genomic diseases.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
JULY 18, 2023
Scribe Therapeutics and Sanofi have expanded partnership to progress the development of in vivo genetic therapies to treat genomic diseases.
pharmaphorum
JUNE 28, 2021
The first ever clinical data with a CRISPR/Cas9 drug used to edit the genomes of cells within the body has yielded impressive results in patients with ATTR amyloidosis, a life-threatening rare disease. . — Eric Topol (@EricTopol) June 26, 2021. — Eric Topol (@EricTopol) June 26, 2021.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioSpace
MARCH 10, 2021
Intellia Therapeutics presented preclinical data of its non-viral genome editing platform at the Keystone eSymposium: Precision Engineering of the Genome, Epigenome and Transcriptome.

pharmaphorum
JANUARY 29, 2021
Genome editing is an exciting but still nascent field, and companies in the area face as many obstacles as they do opportunities. Sangamo CEO Sandy Macrae told us how his company is being cautious about the hype and finding ways to be financially viable in an emerging space. Zinc fingers. billion in funding.
Pharmaceutical Technology
FEBRUARY 15, 2023
Innovation S-curve for the pharmaceutical industry CRISPR nuclease is a key innovation area in pharmaceutical development CRISPR, which refers to clustered regularly interspaced short palindromic repeats, are bacteriophage-derived DNA sequences that had previously infected the prokaryote and are found in the genomes of bacteria and archaea.

Pharmaceutical Technology
MAY 17, 2023
Scribe Therapeutics has entered a strategic collaboration with Eli Lilly and Company subsidiary Prevail Therapeutics for accelerating in vivo CRISPR-based therapies to target the causes of serious neurological and neuromuscular diseases. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.

BioSpace
AUGUST 31, 2021
On June 26, Intellia announced the first-ever clinical data supporting the safety and efficacy of in vivo CRISPR genome editing in human patients.

Pharmaceutical Technology
FEBRUARY 23, 2023
Moderna has entered a strategic research and development partnership with ElevateBio’s Life Edit Therapeutics to discover and develop new in-vivo mRNA gene editing therapies. The company’s nuclease collection includes several Protospacer Adjacent Motifs (PAMs), short sequences that help determine the genome’s DNA segments.

pharmaphorum
JUNE 23, 2022
Novartis has shouldered its way into the in vivo gene editing category via a deal with US biotech Precision BioSciences, focused on a therapy for sickle cell disease (SCD). Precision Bio aims to address those challenges using its proprietary ARCUS nuclease platform, which is designed to insert a transgene with high accuracy into the genome.
Pharmaceutical Technology
SEPTEMBER 29, 2022
The alliance will leverage the CRISPR genome editing technologies of Scribe to facilitate in genetic modification of new natural killer (NK) cell therapeutics for cancer. Under the agreement, Sanofi will receive non-exclusive rights to Scribe’s CRISPR by Design platform of wholly-owned enzymes for developing ex vivo NK cell therapies.

BioTech 365
MAY 11, 2021
New Preclinical Data Presented at the 2021 American Society of Genetic & Cell Therapy Annual Meeting Highlights Precision BioSciences’ ARCUS In Vivo Genome Editing New Preclinical Data Presented at the 2021 American Society of Genetic & Cell Therapy Annual Meeting … Continue reading →

Pharmaceutical Technology
FEBRUARY 23, 2023
DNA binding site prediction, peptide structure optimisation, and AI-assisted genome analysis are some of the accelerating innovation areas, where adoption has been steadily increasing. However, not all innovations are equal nor do they follow a constant upward trend.

Camargo
NOVEMBER 11, 2020
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. Unpacking the (Black) Box: Antares Licenses Urology Product with Boxed Warning. of hyponatremia, or low blood sodium levels. In October, the U.S.

Pharmaceutical Technology
FEBRUARY 15, 2023
They are engineered to cut specific genomic targets in order to modify the expression of single genes and proteins. In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Gene splicing using nucleases.

Roots Analysis
FEBRUARY 27, 2024
The global genome editing market is anticipated to grow at a CAGR of 12.6% The global genome editing market is anticipated to grow at a CAGR of 12.6% Recently, in July 2019, a first in vivo clinical trial for a CRISPR-based therapy was initiated. during the forecast period 2023-2035. during the forecast period 2023-2035.
Pharmaceutical Technology
FEBRUARY 14, 2023
These systems include human and mouse cell lines, and even in vivo in live animals. These systems include human and mouse cell lines, and even in vivo in live animals. However, not all innovations are equal and nor do they follow a constant upward trend.
Scienmag
NOVEMBER 16, 2020
Wnt-regulated lncRNA discovery enhanced by in vivo identification and CRISPRi functional validation. Genome Med 12, 89 (2020). Singapore scientists uncover potential role of long non-coding RNAs in pancreatic cancer Credit: From Figure 4 in Liu, S., Harmston, N., Glaser, T.L.

pharmaphorum
JULY 13, 2022
Verve Therapeutics has started dosing patients in a phase 1b trial of its in vivo gene-editing drug for high cholesterol, designed to permanently switch off the PCSK9 gene with a one-shot treatment. dosing of *first patient* with VERVE-101, an in vivo CRISPR base editing medicine. Today: we are announcing.

Pharmaceutical Technology
MARCH 9, 2023
Innovation S-curve for the pharmaceutical industry Transgenic murine models is a key innovation area in pharmaceutical s Transgenic murine models refer to mice that have been genetically altered for the purposes of understanding the in vivo functions of genes.

The Pharma Data
DECEMBER 13, 2020
Nasdaq: DTIL) a clinical stage biotechnology company dedicated to improving life with its novel and proprietary ARCUS® genome editing platform, today announced that Abid Ansari, Chief Financial Officer, notified the Company that he will be leaving the organization after nearly five years to pursue a new career opportunity. “On DURHAM, N.C.,

pharmaphorum
OCTOBER 6, 2022
Disease modelling Gulf War Illness. In the mid-to-late 1990s, an unexplained illness began to emerge in army bases across the US. Symptoms and their severity ranged across individuals, and included fatigue, musculoskeletal pain, skin rashes, headaches, and gastrointestinal issues. Tinker, tailor, soldier, therapy.

XTalks
AUGUST 5, 2020
Rosha Poudyal, PhD, Science and Technology Advisor at 10x Genomics, discussed some of the innovative single cell technology tools that the company is developing and their application in various research areas including oncology, infectious disease and immunology. The Power of Single Cell Technology. Feature barcoding using gel beads.

XTalks
DECEMBER 20, 2023
As we step into 2024, the life sciences continue to evolve at an unprecedented pace, driven by technological innovation, a deeper understanding of human biology and the application of new technologies in areas like drug development and health wearables. Regulatory bodies are also taking note of the applications of AI in drug development.

XTalks
MAY 22, 2024
John Finn, PhD Chief Scientific Officer Tome Biosciences Dr. Finn has over 20 years of experience in the gene therapy space with a focus on genome editing and delivery technologies. PGI is a cutting-edge gene editing technology that allows for the insertion of large sequences of DNA with site-specific precision.

The Pharma Data
DECEMBER 13, 2020
which develops genome editing technologies to accelerate drug discovery and develop novel therapeutics for a broad range of diseases, today announced the appointment of Bo Zhang, Ph.D., 14, 2020 10:00 UTC. BEIJING & CAMBRIDGE, Mass.–( –( BUSINESS WIRE )– EdiGene, Inc. as Head of the US Subsidiary, and Kehua Fan, M.D.,

pharmaphorum
OCTOBER 8, 2020
Doudna is serving as scientific advisor to Scribe, and its chief executive is Benjamin Oakes, who has been working on genome editing for most of his scientific career, starting out looking at a rival technology to CRISPR called zinc finger nucleases before working in Doudna’s lab at the University of California, Berkeley. Benjamin Oakes.
The Pharma Data
NOVEMBER 22, 2020
Back in September, Vertex Pharmaceuticals and CRISPR Therapeutics – the company behind the CRISPR Cas-9 platform – announced that the European Medicines Agency (EMA) had granted Priority Medicines (PRIME) designation to CTX001, an investigational ex vivo CRISPR Cas-9 gene-edited therapy for the treatment of severe sickle cell disease.

The Pharma Data
JANUARY 12, 2021
KSQ’s proprietary CRISPRomics® discovery engine enables genome-scale, in vivo validated, unbiased drug discovery across broad therapeutic areas. KSQ was founded by thought leaders in the field of functional genomics and pioneers of CRISPR screening technologies. and retain royalties on all ex-U.S. sales for that product.

The Pharma Data
JANUARY 10, 2021
Nasdaq: EDIT), a leading genome editing company, today announced the U.S. EDIT-301 is an experimental, ex vivo gene editing cell medicine in development for the treatment of sickle cell disease. CAMBRIDGE, Mass., 11, 2021 (GLOBE NEWSWIRE) — Editas Medicine, Inc.

The Pharma Data
JUNE 7, 2023
Acuitas’ LNP technology will support Bayer’s in vivo gene editing and protein replacement programs by specifically delivering RNA payloads to the desired target organ, the liver. a biotechnology company specializing in the development of lipid nanoparticle (LNP) delivery systems for molecular therapeutics. Source link: [link]

The Pharma Data
DECEMBER 1, 2020
NASDAQ:NTLA), a leading genome editing company focused on developing curative therapeutics using CRISPR/Cas9 technology both in vivo and ex vivo , announced today the pricing of an underwritten public offering of 4,794,521 shares of its common stock at a public offering price of $36.50 CAMBRIDGE, Mass., Goldman Sachs & Co.

Delveinsight
FEBRUARY 25, 2021
Instead of snipping the genome, base editing lets for edits of individual letters in a genetic sequence. Electroporation is used for ex vivo delivery of therapies to blood and immune cells. Beam announced that it paid USD 120 million upfront to acquire Guide Therapeutics in an all-stock agreement.

Pharma Marketing Network
DECEMBER 21, 2020
Almost two decades after the human genome was sequenced, a trickle of new genetic medicines (i.e., those that modify the expression of an individual’s genes or repair abnormal genes) has entered clinical practice, including 11 RNA therapeutics, 2 in vivo gene therapies, and 2 gene-modified cell therapies.

Pharma Marketing Network
JANUARY 20, 2021
In other areas of the industry (such as cell and genetic medicine development) progress continued at an impressively brisk pace despite the pandemic. Regarding OWS’s support for vaccine development, Dr Slaoui noted that 5 of the 6 vaccines selected (from a total of 94 programs) are currently in phase 3 development or approved.

XTalks
AUGUST 12, 2020
Despite the effectiveness of anti-retroviral therapy (ART) in significantly improving health, quality of life and reducing mortality among HIV-positive individuals, new research shows that persistent infections may be due to the ability of the virus to take refuge in distinct subsets of immune T cells.

The Pharma Data
JANUARY 4, 2021
Kiromic chPD1 has shown in preclinical data to show a cytotoxic response in 9 different in vivo models with 100% long-term PFS with the induction of host memory responses. chPD1 will be used in the Company’s proprietary chimeric antigen receptor therapy (CAR-T) platform using gamma-delta T-cells (GD-T). About Kiromic.

XTalks
OCTOBER 20, 2023
Clinical-stage genome editing company Intellia Therapeutics has received clearance from the US Food and Drug Administration (FDA) for its Investigational New Drug (IND) application to start a pivotal phase III trial of NTLA-2001 for the treatment of transthyretin (ATTR) amyloidosis with cardiomyopathy.

XTalks
NOVEMBER 3, 2023
CRISPR works as genetic scissors to edit parts of the genome. The companies used data from the 1,000 Genomes Project but from that, only 61 datasets made the cut to encompass the ideal patient population. “I The CRISPR-Cas9 gene editing system was first discovered to be endogenous in bacteria.

The Pharma Data
DECEMBER 2, 2020
” VANCOUVER, BC, December 02, 2020 /24-7PressRelease/ — Eyam Vaccines and Immunotherapeutics (EYAM) today announced that former President and CEO of Genome Prairie is joining EYAM. Dr. Pontarollo’s primary areas of research and technical expertise include Genomics, Molecular Biology, Vaccine development and Immunology.

The Pharma Data
OCTOBER 14, 2020
VBL has recently demonstrated ex-vivo activity of anti-MOSPD2 antibodies in patients with relapsing-remitting and progressive multiple sclerosis (MS), as well as in animal models of rheumatoid arthritis (RA), nonalcoholic steatohepatitis (NASH) and inflammatory bowel disease (IBD). 1. -- -->. -- [if lte IE 8]--> .

XTalks
APRIL 24, 2023
Rare diseases can often be progressive, chronic and fatal. Approximately 72 percent of rare diseases are genetic, and around 70 percent of rare genetic diseases emerge in childhood. Sadly, one-third of children with rare diseases die before their first birthday.
The Pharma Data
DECEMBER 10, 2020
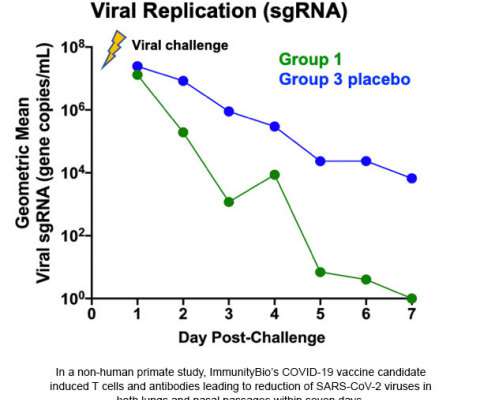
.–( BUSINESS WIRE )– ImmunityBio , a privately-held immunotherapy company, today announced its COVID-19 vaccine candidate protected nasal and lung airways of non-human primates against coronavirus (SARS-CoV-2) in a challenge study. This blocking of viral replication was observed in both the lung and nasal passages.

FDA Law Blog
DECEMBER 6, 2024
There is even a tip of the hat to alternative (non-animal) test methods, which have become powerful methods for the assessment of the potential for off-target toxicity and unintended genome editing. Section #1: FDA Interactions Given the wide range of sponsors (i.e., CBER will not commit to reviewing packages greater than 250 pages.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content