Pfizer/BioNTech Begin Studies for Third Booster Dose and Development of COVID-19 Vaccine Variants
XTalks
MARCH 1, 2021
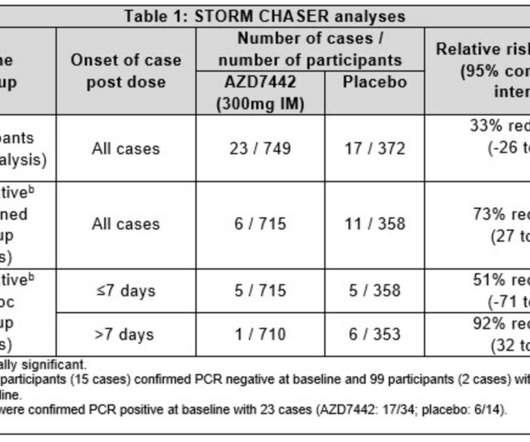
(NYSE: PFE ) and BioNTech SE (Nasdaq: BNTX ) have announced that they are beginning a trial to evaluate the safety and efficacy of a third booster dose for their COVID-19 vaccine (BNT162b2), as well as new vaccine variants. Related: Could Pfizer and BioNTech’s COVID-19 Vaccine be Stored at Standard Freezer Temperatures?






























Let's personalize your content