Positive in vitro results for Imutex’s FLU-v
Pharma Times
OCTOBER 1, 2022
Data further supports the continued development of FLU-v as a broad-spectrum influenza vaccine
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharma Times
OCTOBER 1, 2022
Data further supports the continued development of FLU-v as a broad-spectrum influenza vaccine

World of DTC Marketing
JULY 12, 2021
SUMMARY: Pfizer says it has data on waning immunity from their COVID vaccine. According to a report on CNN.com “Pfizer said it is seeing waning immunity from its coronavirus vaccine and says it is picking up its efforts to develop a booster dose that will protect people from variants.” ” So who to believe?
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioPharma Reporter
JANUARY 28, 2021
In vitro studies found the Pfizer/BioNTech COVID-19 vaccine elicits antibodies against both the UK and South African virus strains.

Pharmaceutical Technology
MAY 12, 2023
BioNTech has ended its research collaboration with Matinas after its oral mRNA vaccine failed to demonstrate preclinical activity. Whilst the formulation had been successful in vitro , the oral administration in mice did not elicit activity. Matinas announced the news in a May 10 statement.

BioPharma Reporter
JANUARY 25, 2021
An in vitro study shows Moderna's existing mRNA COVID-19 vaccine provides protection against strains that have emerged from South Africa and the UK. But it does suggest reduced protection against the South Africa strain, and so the company is also developing a new booster designed for maximum efficacy against this variant.
Pharma Mirror
APRIL 21, 2024
With the rapid development of biotechnology and molecular medicine, the introduction of mRNA as a vaccine or therapeutic agent enables the production of almost any desired functional protein/peptide within the human body.

XTalks
MARCH 1, 2021
(NYSE: PFE ) and BioNTech SE (Nasdaq: BNTX ) have announced that they are beginning a trial to evaluate the safety and efficacy of a third booster dose for their COVID-19 vaccine (BNT162b2), as well as new vaccine variants. Related: Could Pfizer and BioNTech’s COVID-19 Vaccine be Stored at Standard Freezer Temperatures?

XTalks
JANUARY 15, 2021
Ortho Clinical Diagnostics received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for its VITROS SARS-CoV-2 antigen test for detection of active COVID-19 infection. Related: COVID-19 Vaccine and Drug Development Coverage. active virus).

Scienmag
JANUARY 25, 2022
Vaccination against COVID-19 did not affect fertility outcomes in patients undergoing in-vitro fertilization (IVF), a new study has found. The findings, which were published in Obstetrics & Gynecology (the Green Journal), add to the growing body of evidence providing reassurance that COVID-19 vaccination does not affect fertility.

Scienmag
DECEMBER 11, 2021
Published today, one of the earliest, peer-reviewed studies looking into the Omicron variant of COVID-19 suggests that people previously infected with COVID, and those vaccinated, will have some, “stronger than basic” defence against this new strain of concern.
Pharmaceutical Technology
JUNE 1, 2023
The vaccine is self-administered through an intranasal spray, and is based on the company’s GlycoTarge platform. In April 2020, Pneumagen announced positive anti-viral activity results from three in vitro studies into preventing coronavirus infections, but the program has not yet been studied in humans. million ($4.75
Pharmaceutical Technology
NOVEMBER 22, 2022
Vaccines are our number one weapon in the fight against infectious diseases, but their development has historically involved a long and complex process taking up to a decade. Before COVID-19, Merck held the record for the fastest modern vaccine ever developed. mRNA’s potential for rapid vaccine delivery.
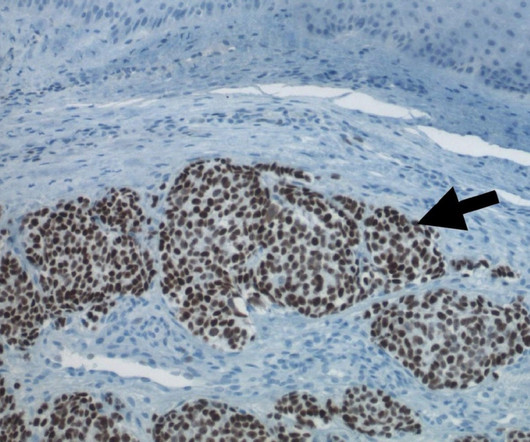
Pharmaceutical Technology
MAY 24, 2023
Morphogenesis’s technologies include Immune Fx (IFx) personalised cancer vaccines and tumour microenvironment (TME) modulators. Morphogenesis’ lead personalised cancer vaccine, IFx-Hu2.0, Morphogenesis’ lead personalised cancer vaccine, IFx-Hu2.0, The company is also advancing its mRNA vaccine, IFx-Hu3.0,

pharmaphorum
JANUARY 22, 2021
So far, neither variant seems to be associated with more severe COVID-19 symptoms, although there has been some preliminary research suggesting the SA strain could allow reinfection with SARS-CoV-2, and also be less susceptible to vaccines. The UK strain – known as B.1.1.7

XTalks
APRIL 27, 2022
In the US, the vaccination for the virus is currently provided to individuals five years of age and older. The in vitro studies showed that remdesivir was effective among the drugs because it had the strongest antiviral activity as it could block virus infection at low micromolar concentrations with minimal cell toxicity.

Camargo
NOVEMBER 29, 2021
Section 351 of the Public Health Service (PHS) Act defines a biological product as a “virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, or analogous product. Biologics include a wide range of products , including: Vaccines. Definition of Biologic Products. Allergenics.
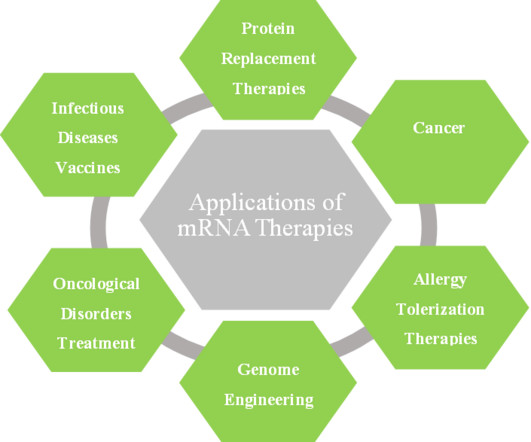
Roots Analysis
MARCH 16, 2023
In the last few years, researchers have become interested in using in vitro transcribed (IVT) mRNA as a drug delivery agent. It is worth noting that several companies have begun to develop mRNA-based cancer immunotherapies and vaccines for infectious diseases.

Pfizer
JANUARY 27, 2023
In the ongoing development of the Pfizer-BioNTech COVID-19 vaccine, Pfizer has not conducted gain of function or directed evolution research. This research provides a way for us to rapidly assess the ability of an existing vaccine to induce antibodies that neutralize a newly identified variant of concern. In addition, to meet U.S.
The Pharma Data
AUGUST 15, 2021
Even as several safe and effective COVID-19 vaccines are being administered to people worldwide, scientists are still hard at work developing different vaccine strategies that could provide even stronger or longer-lasting immunity against SARS-CoV-2 and its variants.

pharmaphorum
DECEMBER 16, 2020
Soon the ABPI and the entire industry were facing the ripple effects of COVID-19, including a significant slowdown in non-COVID clinical research, which Torbett says was challenging for the industry to deal with. “We’ve We’ve also seen a very mixed picture in the market for pharmaceuticals,” he says. Strength in the second wave.

Pharmaceutical Technology
SEPTEMBER 10, 2008
Secondly, we conduct pre-clinical research and development in the fields of oncology, cardiovascular disorders, anti-influenza vaccines and diabetology. IS: Our genotoxicity testing includes the core battery of the tests requested by EMEA/ICH including mutagenicity in vitro, chromosomal aberration test in vitro and micronucleus test in vivo.

pharmaphorum
JANUARY 17, 2023
Research into mRNA dates back to the 1970s, but with the approval of both Moderna and BioNTech/Pfizer’s vaccines, this approach has finally been validated. At the end of last year, the UK government entered into a partnership with the company, which saw Moderna agree to provide 250 million vaccine doses per year over the course of a decade.

The Pharma Data
SEPTEMBER 17, 2020
This is the focus of vaccines in development and convalescent plasma therapy. The majority of current candidate vaccines aim to induce an antibody response against the spike protein. “As The test targets antibodies against the spike protein. Food and Drug Administration (FDA). said Thomas Schinecker, CEO Roche Diagnostics.

XTalks
MARCH 27, 2024
In vitro diagnostics, or IVD, is a field where precision, reliability and robust partnerships propel advancement. Dr. Dhiman has 24 years of clinical research experience in the immunogenetics of vaccines and infectious disease diagnostics. She did her clinical microbiology fellowship at Mayo Clinic in Rochester, Minnesota.

XTalks
JUNE 27, 2022
CerTest Biotec specializes in the development and manufacturing of in vitro diagnostic medical devices and has expertise in the detection of human diseases. As such, the smallpox vaccine can be used to protect against monkeypox — according to the WHO, the vaccine is 85 percent effective against monkeypox infection.
pharmaphorum
OCTOBER 5, 2022
It has been shown in vitro to cover all major influenza A strains that have arisen since the 1918 ‘Spanish flu’ pandemic and is designed as a universal prophylactic for influenza A. VIR-2482 is an investigational intramuscularly administered influenza A-neutralising mAb.
pharmaphorum
NOVEMBER 4, 2020
However, in vitro or binding-based efficacy insight needs to be confirmed by reliable clinical evidence, as shown by the outcomes of COVID-19 trials with remdesivir and hydroxychloroquine. million worldwide and continues to increase despite the global public health measures that have been put in place.

The Pharma Data
MAY 17, 2021
The European Commission’s Medical Device Coordination Group (MDCG) sent a notice to in vitro diagnostic (IVD) makers and their authorized representatives reminding them of their obligation to assess the impact of COVID-19 variants on the performance of their tests. . Posted 17 May 2021 | By Michael Mezher .

Scienmag
SEPTEMBER 18, 2020
Antibody-like proteins that capture and neutralize SARS-CoV-2 Scientists have used a new high-speed, in vitro selection method to isolate 9 antibody-like proteins (ALPs) that bind to the SARS-CoV-2 virus – 4 of which also exhibited neutralizing activity – within 4 days, according to a new study.

XTalks
SEPTEMBER 30, 2020
The two studies were recently published online in the journal Science, both of which were led by Jean-Laurent Casanova, an infectious disease geneticist at Rockefeller University. The findings reveal a critical role for IFNs in fighting COVID-19 infection, positioning them to be a potential therapeutic target in the disease. percent of women and 12.5

pharmaphorum
JANUARY 16, 2023
The present article focuses on opportunity, the unmet need which lies at the heart of better healthcare provision, and two areas of growth and opportunity: Point of Care Diagnostics, and the new pharmacotherapy classes which will commercialise for the first time in 2023. The hard problem: innovation for high prevalence, chronic diseases of ageing.

pharmaphorum
AUGUST 4, 2022
If not internally, such specialist training programmes can be outsourced to third-party organisations, whether that involves pharmaceuticals, in vitro diagnostics (IVDs) or QP training. This has resulted in staff and talent shortages, meaning that industries have been forced to respond quickly to stay afloat. Harness technology.

Pfizer
AUGUST 15, 2022
I am grateful to have received four doses of the Pfizer-BioNTech vaccine and I am feeling well while experiencing very mild symptoms. I am incredibly grateful for the tireless efforts of my Pfizer colleagues who worked to make vaccines and treatments available for me and people around the world.

The Pharma Data
JANUARY 11, 2021
Testing Therapies, Antivirals and Vaccines. By the end of January, Johnson & Johnson is expected to deliver preliminary trial results for its one-dose COVID-19 vaccine candidate. . One of the open questions about the various vaccines against COVID-19 has been how long the protection offered will last. Diagnostics.

The Pharma Data
MARCH 23, 2021
The oral antiviral clinical candidate PF-07321332, a SARS-CoV2-3CL protease inhibitor , has demonstrated potent in vitro anti-viral activity against SARS-CoV-2, as well as activity against other coronaviruses, suggesting potential for use in the treatment of COVID-19 as well as potential use to address future coronavirus threats.

Pharmaceutical Technology
AUGUST 26, 2008
It was once derided as the stuff of science fiction, but in recent years, biotechnology has emerged as an important growth area in pharmaceuticals. As understanding of biological systems has forged ahead, pharmaceutical companies have made increasing use of biotechnology in discovering and manufacturing new medicines.
Delveinsight
FEBRUARY 23, 2021
The company develops transformative biologics including vaccines and therapeutics for the world’s most debilitating diseases. The company plans to use the capital to expand its pipeline of protein-based vaccines and biologic cancer therapies utilizing its innovative and proprietary Trimer-Tag technology platform.

Pfizer
DECEMBER 13, 2022
Nirmatrelvir has shown consistent in vitro antiviral activity against the following variants: Alpha, Beta, Delta, Gamma, Lambda, Mu, and Omicron BA.1, The additional 3.7 million treatment courses are planned for delivery by early 2023. . In June of 2022, Pfizer submitted a New Drug Application (NDA) to the U.S.
XTalks
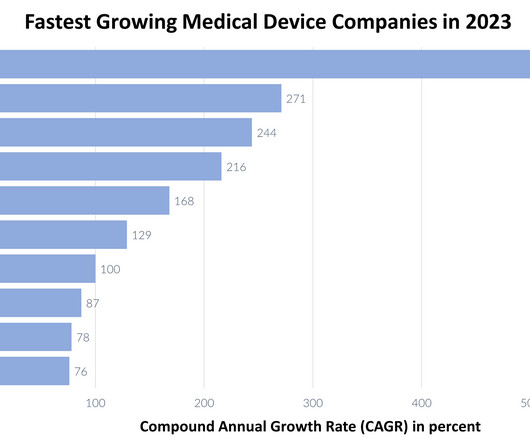
DECEMBER 11, 2023
Medical device companies continue to maintain a crucial role in enhancing patient care and diagnostic precision in 2023. Let’s examine the list of the top ten fastest growing medical device companies in 2023, ranked by their compound annual growth rate (CAGR). In 2021, Axonics achieved an impressive revenue of $180.3
Roots Analysis
AUGUST 15, 2023
Overview of Cell Free Systems Cell-free systems are in-vitro platforms which allow occurrence of biochemical reactions in the absence of living cells. These expression systems utilize bio machinery harvested from the lysate of disrupted cells for the manufacturing of a wide array of macromolecular and small molecule products.
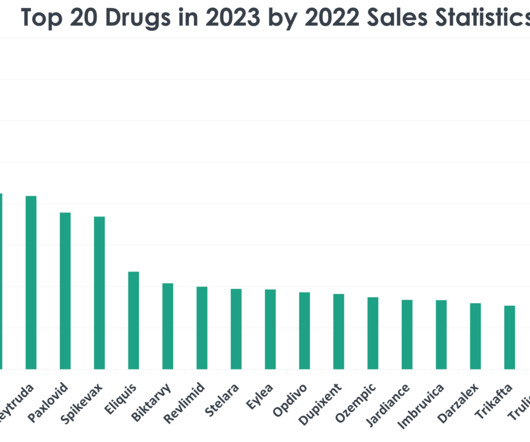
XTalks
DECEMBER 19, 2023
1) Comirnaty (COVID-19 Vaccine, mRNA) Sales in 2022: $37.81 Why it did so well: The COVID-19 pandemic was one of the major drivers for Comirnaty sales in the US in 2022 as it remained the vaccine of choice throughout the pandemic. The rise of the Omicron variant fuelled the sale of the vaccine. billion in sales in 2022.

The Pharma Data
MARCH 26, 2021
New in vitro data from pseudotyped virus assays published online in bioRxiv in March 2021 support this hypothesis as they demonstrate that VIR-7831 maintains activity against current circulating variants of concern including the UK, South African and Brazilian variants. About the VIR-7831 Clinical Development Programme.
The Pharma Data
AUGUST 22, 2021
AZD7442 is the first antibody combination (non-vaccine) modified to potentially provide long-lasting protection that has demonstrated prevention of COVID-19 in a clinical trial. More than 75% of participants had co-morbidities, which include conditions that have been reported to cause a reduced immune response to vaccination.

The Pharma Data
DECEMBER 20, 2020
Food and Drug Administration (FDA) is plenty busy with COVID-19 vaccine Emergency Use Authorizations (EUAs) this month, but they’re also wrapping up the year with a few PDUFA dates for other therapies. Here’s a look. Urovant Sciences’ Vibegron for Overactive Bladder. The drug is a once-daily, beta-3 adrenergic agonist.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content