Positive in vitro results for Imutex’s FLU-v
Pharma Times
OCTOBER 1, 2022
Data further supports the continued development of FLU-v as a broad-spectrum influenza vaccine
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharma Times
OCTOBER 1, 2022
Data further supports the continued development of FLU-v as a broad-spectrum influenza vaccine

World of DTC Marketing
JULY 12, 2021
SUMMARY: Pfizer says it has data on waning immunity from their COVID vaccine. According to a report on CNN.com “Pfizer said it is seeing waning immunity from its coronavirus vaccine and says it is picking up its efforts to develop a booster dose that will protect people from variants.” ” So who to believe?
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioPharma Reporter
JANUARY 28, 2021
In vitro studies found the Pfizer/BioNTech COVID-19 vaccine elicits antibodies against both the UK and South African virus strains.

Pharmaceutical Technology
SEPTEMBER 19, 2022
AstraZeneca Vaccines and Immune Therapies executive vice-president Iskra Reic said: “Evusheld has already made an important difference around the world helping prevent Covid-19 infections in vulnerable populations who can’t mount an adequate response to Covid-19 vaccination. 5 variant, the company noted. 5 variant, the company noted.

Pharmaceutical Technology
MAY 12, 2023
BioNTech has ended its research collaboration with Matinas after its oral mRNA vaccine failed to demonstrate preclinical activity. Whilst the formulation had been successful in vitro , the oral administration in mice did not elicit activity. Matinas announced the news in a May 10 statement.

BioPharma Reporter
JANUARY 25, 2021
An in vitro study shows Moderna's existing mRNA COVID-19 vaccine provides protection against strains that have emerged from South Africa and the UK. But it does suggest reduced protection against the South Africa strain, and so the company is also developing a new booster designed for maximum efficacy against this variant.

XTalks
MARCH 1, 2021
(NYSE: PFE ) and BioNTech SE (Nasdaq: BNTX ) have announced that they are beginning a trial to evaluate the safety and efficacy of a third booster dose for their COVID-19 vaccine (BNT162b2), as well as new vaccine variants. Related: Could Pfizer and BioNTech’s COVID-19 Vaccine be Stored at Standard Freezer Temperatures?
pharmaphorum
MARCH 4, 2021
UK chancellor Rishi Sunak has announced a budget loaded with initiatives designed to kick-start the UK’s economy as it recovers from the coronavirus pandemic, with vaccine development, pharma and life sciences playing a key role. A scheme providing £500 payments to people self-isolating has been extended in England until the summer.
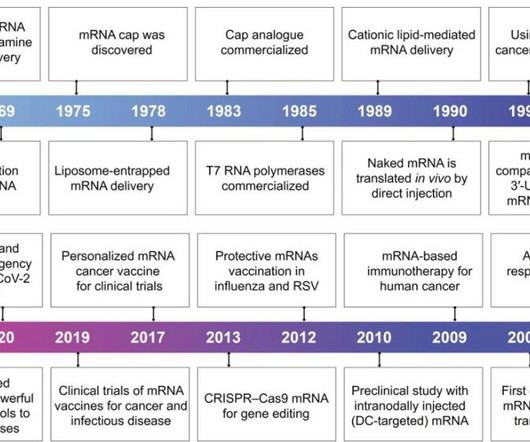
Pharma Mirror
APRIL 21, 2024
With the rapid development of biotechnology and molecular medicine, the introduction of mRNA as a vaccine or therapeutic agent enables the production of almost any desired functional protein/peptide within the human body.

Scienmag
JANUARY 25, 2022
Vaccination against COVID-19 did not affect fertility outcomes in patients undergoing in-vitro fertilization (IVF), a new study has found. The findings, which were published in Obstetrics & Gynecology (the Green Journal), add to the growing body of evidence providing reassurance that COVID-19 vaccination does not affect fertility.
Pharmaceutical Technology
NOVEMBER 22, 2022
Vaccines are our number one weapon in the fight against infectious diseases, but their development has historically involved a long and complex process taking up to a decade. Before COVID-19, Merck held the record for the fastest modern vaccine ever developed. mRNA’s potential for rapid vaccine delivery.

Scienmag
DECEMBER 11, 2021
Published today, one of the earliest, peer-reviewed studies looking into the Omicron variant of COVID-19 suggests that people previously infected with COVID, and those vaccinated, will have some, “stronger than basic” defence against this new strain of concern.

XTalks
JANUARY 15, 2021
Ortho Clinical Diagnostics received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for its VITROS SARS-CoV-2 antigen test for detection of active COVID-19 infection. Related: COVID-19 Vaccine and Drug Development Coverage. active virus).
XTalks
NOVEMBER 15, 2023
France-based biotech Valneva has won approval from the US Food and Drug Administration (FDA) for its chikungunya vaccine Ixchiq for the prevention of infection from the chikungunya virus. The vaccine is approved for adults 18 years of age and older who are at increased risk of exposure to the virus.

XTalks
MARCH 11, 2021
Amidst initial confusion, fear and chaos, masks and social distancing quickly became new norms, and now vaccines are leading hope for a way out. With vaccine shortages and slow rollouts in many parts of the world, the COVID-19 way of life continues in many places. As of March 11, 2021, COVID-19 has claimed the lives of over 2.6

XTalks
APRIL 2, 2021
Pfizer released new COVID-19 vaccine trial results this week from its ongoing clinical studies, which include data showing that its COVID-19 mRNA vaccine is 100 percent effective in children between the ages of 12 and 15 and has a 91 percent efficacy against variants with protection lasting at least six months.
XTalks
JANUARY 27, 2021
COVID-19 vaccine maker Moderna announced this week that results from a study show that the shot is effective against some of the new circulating variants of SARS-CoV-2. In a news release from Moderna, the company reported that the administration of its COVID-19 vaccine induced antibody titers against both variants.
Pharmaceutical Technology
MAY 24, 2023
Morphogenesis’s technologies include Immune Fx (IFx) personalised cancer vaccines and tumour microenvironment (TME) modulators. Morphogenesis’ lead personalised cancer vaccine, IFx-Hu2.0, Morphogenesis’ lead personalised cancer vaccine, IFx-Hu2.0, The company is also advancing its mRNA vaccine, IFx-Hu3.0,
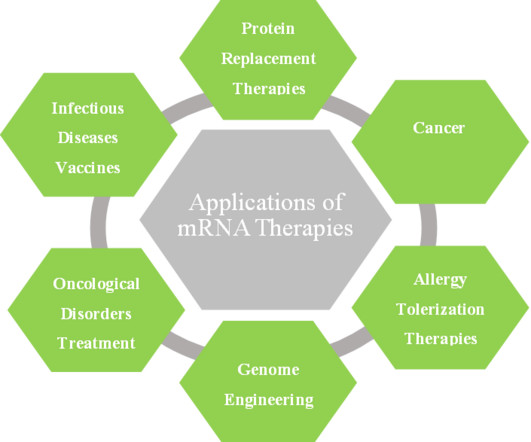
Roots Analysis
MARCH 16, 2023
In the last few years, researchers have become interested in using in vitro transcribed (IVT) mRNA as a drug delivery agent. It is worth noting that several companies have begun to develop mRNA-based cancer immunotherapies and vaccines for infectious diseases.
Pharmaceutical Technology
JUNE 1, 2023
The vaccine is self-administered through an intranasal spray, and is based on the company’s GlycoTarge platform. In April 2020, Pneumagen announced positive anti-viral activity results from three in vitro studies into preventing coronavirus infections, but the program has not yet been studied in humans. million ($4.75
The Pharma Data
MARCH 11, 2021
(NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced real-world evidence demonstrating dramatically lower incidence rates of COVID-19 disease in individuals fully vaccinated with the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2), underscoring the observed substantial public health impact of Israel’s nationwide immunization program.

Pfizer
JANUARY 27, 2023
In the ongoing development of the Pfizer-BioNTech COVID-19 vaccine, Pfizer has not conducted gain of function or directed evolution research. This research provides a way for us to rapidly assess the ability of an existing vaccine to induce antibodies that neutralize a newly identified variant of concern. In addition, to meet U.S.
pharmaphorum
OCTOBER 18, 2021
The development of COVID-19 vaccines required levels of cooperation and pioneering science comparable to the Apollo space programme. Within 12 months of the outbreak, vaccines were being deployed to prevent severe infections, hospitalisation, and death. billion people have been fully vaccinated. And there are many.
The Pharma Data
AUGUST 15, 2021
Even as several safe and effective COVID-19 vaccines are being administered to people worldwide, scientists are still hard at work developing different vaccine strategies that could provide even stronger or longer-lasting immunity against SARS-CoV-2 and its variants.

XTalks
APRIL 27, 2022
In the US, the vaccination for the virus is currently provided to individuals five years of age and older. The in vitro studies showed that remdesivir was effective among the drugs because it had the strongest antiviral activity as it could block virus infection at low micromolar concentrations with minimal cell toxicity.

pharmaphorum
JANUARY 22, 2021
So far, neither variant seems to be associated with more severe COVID-19 symptoms, although there has been some preliminary research suggesting the SA strain could allow reinfection with SARS-CoV-2, and also be less susceptible to vaccines. The UK strain – known as B.1.1.7

Camargo
NOVEMBER 29, 2021
Section 351 of the Public Health Service (PHS) Act defines a biological product as a “virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, or analogous product. Biologics include a wide range of products , including: Vaccines. Definition of Biologic Products. Allergenics.

pharmaphorum
DECEMBER 16, 2020
Soon the ABPI and the entire industry were facing the ripple effects of COVID-19, including a significant slowdown in non-COVID clinical research, which Torbett says was challenging for the industry to deal with. “We’ve We’ve also seen a very mixed picture in the market for pharmaceuticals,” he says. Strength in the second wave.

The Pharma Data
SEPTEMBER 17, 2020
This is the focus of vaccines in development and convalescent plasma therapy. The majority of current candidate vaccines aim to induce an antibody response against the spike protein. “As The test targets antibodies against the spike protein. Food and Drug Administration (FDA). said Thomas Schinecker, CEO Roche Diagnostics.

pharmaphorum
JANUARY 17, 2023
Research into mRNA dates back to the 1970s, but with the approval of both Moderna and BioNTech/Pfizer’s vaccines, this approach has finally been validated. At the end of last year, the UK government entered into a partnership with the company, which saw Moderna agree to provide 250 million vaccine doses per year over the course of a decade.
pharmaphorum
NOVEMBER 4, 2020
However, in vitro or binding-based efficacy insight needs to be confirmed by reliable clinical evidence, as shown by the outcomes of COVID-19 trials with remdesivir and hydroxychloroquine. million worldwide and continues to increase despite the global public health measures that have been put in place.

Pharmaceutical Technology
SEPTEMBER 10, 2008
Secondly, we conduct pre-clinical research and development in the fields of oncology, cardiovascular disorders, anti-influenza vaccines and diabetology. IS: Our genotoxicity testing includes the core battery of the tests requested by EMEA/ICH including mutagenicity in vitro, chromosomal aberration test in vitro and micronucleus test in vivo.

The Pharma Data
MAY 17, 2021
The European Commission’s Medical Device Coordination Group (MDCG) sent a notice to in vitro diagnostic (IVD) makers and their authorized representatives reminding them of their obligation to assess the impact of COVID-19 variants on the performance of their tests. . Posted 17 May 2021 | By Michael Mezher .

XTalks
JUNE 27, 2022
CerTest Biotec specializes in the development and manufacturing of in vitro diagnostic medical devices and has expertise in the detection of human diseases. As such, the smallpox vaccine can be used to protect against monkeypox — according to the WHO, the vaccine is 85 percent effective against monkeypox infection.
pharmaphorum
OCTOBER 5, 2022
It has been shown in vitro to cover all major influenza A strains that have arisen since the 1918 ‘Spanish flu’ pandemic and is designed as a universal prophylactic for influenza A. VIR-2482 is an investigational intramuscularly administered influenza A-neutralising mAb.

XTalks
MARCH 27, 2024
In vitro diagnostics, or IVD, is a field where precision, reliability and robust partnerships propel advancement. Dr. Dhiman has 24 years of clinical research experience in the immunogenetics of vaccines and infectious disease diagnostics. She did her clinical microbiology fellowship at Mayo Clinic in Rochester, Minnesota.

The Pharma Data
JANUARY 11, 2021
Testing Therapies, Antivirals and Vaccines. By the end of January, Johnson & Johnson is expected to deliver preliminary trial results for its one-dose COVID-19 vaccine candidate. . One of the open questions about the various vaccines against COVID-19 has been how long the protection offered will last. Diagnostics.

Pfizer
AUGUST 15, 2022
I am grateful to have received four doses of the Pfizer-BioNTech vaccine and I am feeling well while experiencing very mild symptoms. I am incredibly grateful for the tireless efforts of my Pfizer colleagues who worked to make vaccines and treatments available for me and people around the world.

The Pharma Data
NOVEMBER 17, 2021
Streamlined in vitro data, published in bioRxiv, demonstrate that sotrovimab retains exertion against all current variants of concern and interest of the SARS-CoV-2 contagion as defined by the World Health Organization, plus others, including, but not limited to, Delta (B.1.617.2), 1.617.2), Delta Plus (AY.1 2) and Mu (B.1.621).

pharmaphorum
AUGUST 4, 2022
If not internally, such specialist training programmes can be outsourced to third-party organisations, whether that involves pharmaceuticals, in vitro diagnostics (IVDs) or QP training. This has resulted in staff and talent shortages, meaning that industries have been forced to respond quickly to stay afloat. Harness technology.

Scienmag
SEPTEMBER 18, 2020
Antibody-like proteins that capture and neutralize SARS-CoV-2 Scientists have used a new high-speed, in vitro selection method to isolate 9 antibody-like proteins (ALPs) that bind to the SARS-CoV-2 virus – 4 of which also exhibited neutralizing activity – within 4 days, according to a new study.

XTalks
SEPTEMBER 30, 2020
The two studies were recently published online in the journal Science, both of which were led by Jean-Laurent Casanova, an infectious disease geneticist at Rockefeller University. The findings reveal a critical role for IFNs in fighting COVID-19 infection, positioning them to be a potential therapeutic target in the disease. percent of women and 12.5

The Pharma Data
MARCH 23, 2021
The oral antiviral clinical candidate PF-07321332, a SARS-CoV2-3CL protease inhibitor , has demonstrated potent in vitro anti-viral activity against SARS-CoV-2, as well as activity against other coronaviruses, suggesting potential for use in the treatment of COVID-19 as well as potential use to address future coronavirus threats.
Delveinsight
FEBRUARY 23, 2021
The company develops transformative biologics including vaccines and therapeutics for the world’s most debilitating diseases. The company plans to use the capital to expand its pipeline of protein-based vaccines and biologic cancer therapies utilizing its innovative and proprietary Trimer-Tag technology platform.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content