Pfizer, BioNTech’s COVID-19 vaccine likely to be effective against UK strain
Pharma Times
JANUARY 21, 2021
Antibodies in blood of vaccinated individuals neutralised in vivo version of variant
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharma Times
JANUARY 21, 2021
Antibodies in blood of vaccinated individuals neutralised in vivo version of variant

Pharmaceutical Technology
MAY 12, 2023
BioNTech has ended its research collaboration with Matinas after its oral mRNA vaccine failed to demonstrate preclinical activity. The in vivo study, conducted in mice, involved a phosphatidylserine-containing nano-formulation of mRNA supplied by BioNTech, distinct from traditional lipid nanocrystals (LNCs).
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
AUGUST 17, 2022
These programmes will include therapies and vaccines in infectious disease and oncology areas. oRNA molecules have been demonstrated to possess increased stability in vivo compared to linear mRNA and can potentially create more quantities of therapeutic proteins within the body. .
pharmaphorum
JANUARY 13, 2021
IO Biotech, an oncology specialist formed and backed by Denmark’s Novo Holdings, has raised €127 million ($155 million) to further develop its cancer vaccine technology that has boosted efficacy of PD-1 immunotherapy in early trials. The post IO Biotech raises $155m to develop breakthrough cancer vaccine appeared first on.

BioTech 365
NOVEMBER 5, 2020
OSE Immunotherapeutics Provides COVID-19 Vaccine Update on CoVepiT, its Multi-Target and Long-Lasting Vaccine Candidate OSE Immunotherapeutics Provides COVID-19 Vaccine Update on CoVepiT, its Multi-Target and Long-Lasting Vaccine Candidate Primary Objective Achieved in Human Ex Vivo Study New Coronavirus Mutated Variants … Continue reading (..)

The Pharma Data
APRIL 22, 2023
The current COVID-19 vaccines are designed to trigger an antibody response to the SARS-CoV-2 spike protein, which is vulnerable to mutations that could make the vaccine less effective over time. of the mice that were vaccinated with the T-cell-based vaccine survived, while only one of the control-group mice survived.
The Pharma Data
DECEMBER 10, 2020
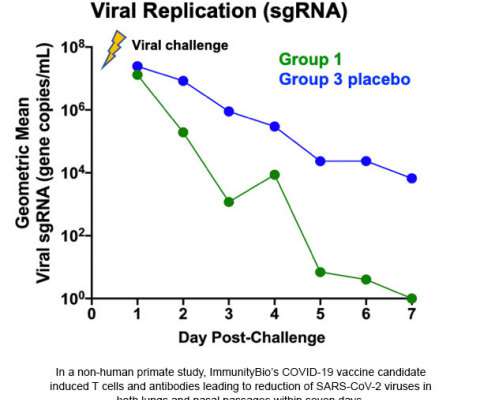
–( BUSINESS WIRE )– ImmunityBio , a privately-held immunotherapy company, today announced its COVID-19 vaccine candidate protected nasal and lung airways of non-human primates against coronavirus (SARS-CoV-2) in a challenge study. CULVER CITY, Calif.–( Graphic: Business Wire).
Pharmaceutical Technology
FEBRUARY 15, 2023
Within the emerging innovation stage, cell therapy for ocular disorders, coronavirus vaccine components, and DNA polymerase compositions are disruptive technologies that are in the early stages of application and should be tracked closely. However, not all innovations are equal and nor do they follow a constant upward trend.

World of DTC Marketing
JANUARY 31, 2021
Ex vivo (where cells are genetically modified outside the body) cell and gene therapies have generated considerable excitement on their potential to cure previously incurable diseases. Still, she is missing a lot of facts at a time when hospital and health insurance costs are soaring. In pharma R&D returns have declined to 1.8

Pharmaceutical Technology
FEBRUARY 15, 2023
Within the emerging innovation stage, cell therapy for ocular disorders, coronavirus vaccine components, and DNA polymerase compositions are disruptive technologies that are in the early stages of application and should be tracked closely. However, not all innovations are equal and nor do they follow a constant upward trend.

Pharmaceutical Technology
MARCH 9, 2023
Within the emerging innovation stage, cell therapy for ocular disorders, coronavirus vaccine components, and DNA polymerase compositions are disruptive technologies that are in the early stages of application and should be tracked closely. However, not all innovations are equal and nor do they follow a constant upward trend.
Pharmaceutical Technology
FEBRUARY 14, 2023
Within the emerging innovation stage, cell therapy for ocular disorders, coronavirus vaccine components, and DNA polymerase compositions are disruptive technologies that are in the early stages of application and should be tracked closely. These systems include human and mouse cell lines, and even in vivo in live animals.
XTalks
OCTOBER 9, 2024
Dr. Culp joined Interius following a distinguished 14-year career with Merck Research Laboratories where he supported vaccine and oncolytic virus program development as a Senior Principal Scientist. He then completed post-doctoral studies at Penn State developing chimeric papillomavirus vaccines.

The Pharma Data
DECEMBER 2, 2020
” VANCOUVER, BC, December 02, 2020 /24-7PressRelease/ — Eyam Vaccines and Immunotherapeutics (EYAM) today announced that former President and CEO of Genome Prairie is joining EYAM. Dr. Pontarollo’s primary areas of research and technical expertise include Genomics, Molecular Biology, Vaccine development and Immunology.

The Pharma Data
NOVEMBER 29, 2020
Early interim data show Cytomegalovirus (CMV)-negative kidney transplant recipients vaccinated with three doses of HB-101 had reduced incidence of CMV viremia, reduced antiviral use and no CMV disease. 21 participants were vaccinated with HB-101 and 12 received placebo. NEW YORK and VIENNA, Austria, Nov.

Pharmaceutical Technology
APRIL 12, 2023
The Association of the British Pharmaceutical Industry (ABPI) also states that “the use of animals in medical research has played and continues to play an essential role in the development of new medicines and vaccines”. This was part of an investigation into an international primate smuggling ring. In 2021, they accounted for 0.2%

BioTech 365
OCTOBER 19, 2020
New in vivo data for colon cancer show anti-tumor immunity in 100% of mice In vivo breast cancer data show a significant delay in challenge tumor uptake GARDEN CITY, N.Y., 19, 2020 (GLOBE NEWSWIRE) — Beyond Air, Inc. NASDAQ: … Continue reading →

Pharmaceutical Technology
SEPTEMBER 10, 2008
Secondly, we conduct pre-clinical research and development in the fields of oncology, cardiovascular disorders, anti-influenza vaccines and diabetology. IS: Our genotoxicity testing includes the core battery of the tests requested by EMEA/ICH including mutagenicity in vitro, chromosomal aberration test in vitro and micronucleus test in vivo.

The Pharma Data
DECEMBER 9, 2020
The Chinese biopharmaceutical industry is growing in leaps and bounds, but there is still a huge unmet need when it comes to getting patients access to the breakthrough therapeutic modalities and platforms like RNAi, cell and gene therapy and others. A solution could be at hand with Overland Pharmaceuticals.

The Pharma Data
JANUARY 17, 2021
Based in Seattle, Washington, Sana focuses on in vivo and ex vivo cell engineering platforms to develop therapies for cancer, diabetes, cardiovascular disease, CNS disorders, and genetic diseases. The Wall Street Journal notes that Moderna, currently one of two companies to have an authorized COVID-19 vaccine in the U.S.,
Pharmaceutical Technology
MAY 24, 2023
Morphogenesis’s technologies include Immune Fx (IFx) personalised cancer vaccines and tumour microenvironment (TME) modulators. Morphogenesis’ lead personalised cancer vaccine, IFx-Hu2.0, Morphogenesis’ lead personalised cancer vaccine, IFx-Hu2.0, The company is also advancing its mRNA vaccine, IFx-Hu3.0,

WCG Clinical
AUGUST 30, 2024
New medications and vaccines will require clinical trials to assess neuropsychological response and imaging endpoints to quantitatively assess amyloid plaque burden and distribution as well as objective changes in regional glucose metabolism. Data from the Alzheimer’s Association estimates that approximately 5.4
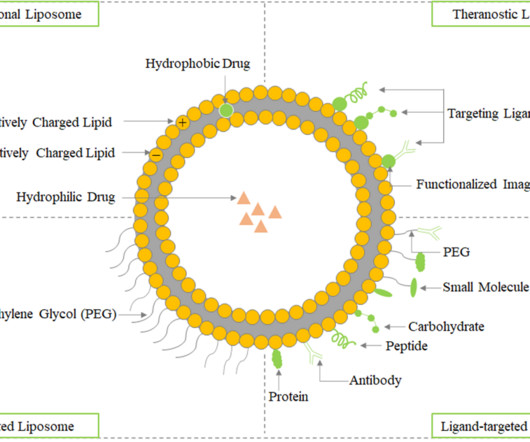
Roots Analysis
AUGUST 8, 2023
Introduction to Liposome Liposome was initially identified and described as swollen phospholipid systems, called Banghasomes, by British hematologist Alec D. Bangham, in 1961 at the Babraham Institute, University of Cambridge. The various therapeutic applications of liposomes in drug delivery have been highlighted in the figure.

XTalks
AUGUST 12, 2020
Xtalks spoke to lead author on the study, Mohamed Abdel-Mohsen, PhD, assistant professor, Vaccine & Immunotherapy Center at the Wistar Institute, about the emerging utility of identifying glycomic, or sugar, signatures on the surface of immune cells to help in their identification and potential targeting.

The Pharma Data
JUNE 17, 2022
Previous research has shown that SARS-CoV-2 spike-specific monoclonal antibodies play a key role in providing in vivo protection and have been used in developing COVID-19 vaccines. A modified version of the Cv2.1169 antibody was also effective at treating SARS-CoV-2 infection in mice and hamsters.
The Pharma Data
DECEMBER 9, 2020
With the expected Emergency Use Authorization (EUA) of Pfizer – BioNTech and Moderna ’s COVID-19 vaccines providing hope that the COVID-19 pandemic will soon be resolved, 2021 is going to need a new primary healthcare campaign. Could it come from the field of neuroscience? which will remain a key shareholder. SciNeuro Pharmaceuticals.

The Pharma Data
MARCH 25, 2022
Despite the roll-out of vaccines and antivirals, the need for effective therapeutics against severe COVID-19 infection remains high. Researchers at Karolinska Institutet in Sweden have developed a novel strategy for identifying potent miniature antibodies, so-called nanobodies, against emerging SARS-CoV-2 variants.

The Pharma Data
OCTOBER 15, 2020
He is currently a professor at the University of Copenhagen where his lab focuses on PNAs in regard to drug discovery, gene targeting, antisense principles, cellular and in vivo delivery and administration of biopharmaceuticals. The Advance awards recognize the work of remarkable global Australians making an extraordinary impact worldwide.

BioTech 365
SEPTEMBER 17, 2020
Positive results for Step-1 of Phase 3 clinical trial of Tedopi® in non-small cell lung cancer Positive preclinical and in human ex vivo results with CoVepiT vaccine against COVID-19; clinical entry expected by the end of 2020 or early 2021 … Continue reading →

Roots Analysis
JANUARY 14, 2024
With the increased interest and gradual shift of investment from small molecule drugs to biologics and the establishment of several biologics manufacturing companies / biologics CMOs, more than 250 biologic therapies and vaccines have been developed, globally. They are different from small molecules in terms of their size and complexity.

The Pharma Data
JANUARY 11, 2021
Xiaomin Fan, Founder, President and CEO of AvantGen, “Even with the recent Emergency Use Authorization of multiple vaccines, we believe there remains an urgent need for potent antibody therapies and prophylactics for immunocompromised patients who cannot mount an adequate immune response either to the vaccine or to subsequent viral infection.

pharmaphorum
JANUARY 17, 2023
Research into mRNA dates back to the 1970s, but with the approval of both Moderna and BioNTech/Pfizer’s vaccines, this approach has finally been validated. At the end of last year, the UK government entered into a partnership with the company, which saw Moderna agree to provide 250 million vaccine doses per year over the course of a decade.

The Pharma Data
JUNE 7, 2023
Acuitas’ LNP technology will support Bayer’s in vivo gene editing and protein replacement programs by specifically delivering RNA payloads to the desired target organ, the liver. a biotechnology company specializing in the development of lipid nanoparticle (LNP) delivery systems for molecular therapeutics. Source link: [link]

The Pharma Data
MARCH 29, 2022
Evusheld has the potential to provide long-lasting protection against COVID-19 for a broad population of individuals, including those who aren’t adequately protected by a COVID-19 vaccine, as well as those at increased risk of exposure.”. 2 subvariant, now the dominant strain in Europe. 1-3 Evusheld was generally well-tolerated in the trial.

The Pharma Data
MARCH 25, 2022
People not adequately protected by a COVID-19 vaccine may particularly benefit from pre-exposure prophylaxis with Evusheld. People not adequately protected by a COVID-19 vaccine may particularly benefit from pre-exposure prophylaxis with Evusheld. 2-4 Evusheld was generally well-tolerated in the trial.(2-4).

The Pharma Data
JANUARY 12, 2021
Nielsen of Vivo Capital and Vanessa Malier of Kurma Partners will join IO Biotech’s board of directors as part of the closing of the financing. IO Biotech offers a unique treatment modality in oncology, harnessing novel immunomodulatory mechanisms through proprietary vaccine candidates. COPENHAGEN, Denmark , Jan.

XTalks
AUGUST 17, 2020
protein-based biologics) and vaccine treatments. This typically involves taking T cells from the body, engineering chimeric receptor antigens (CAR) and inserting them into the cells, expanding the cells ex vivo and injecting them back into the body. Treatment vaccines : these boost the immune system responses to target cells.

Pharma Marketing Network
JANUARY 20, 2021
Regarding OWS’s support for vaccine development, Dr Slaoui noted that 5 of the 6 vaccines selected (from a total of 94 programs) are currently in phase 3 development or approved. The response to this crisis has “transformed the vaccine industry.”.

The Pharma Data
DECEMBER 23, 2020
The European Union (EU) authorized ViiV Healthcare ’s Vocabria (cabotegravir injection and tablets) in conjunction with Janssen’s Edurant (rilpivirine tablets) for the treatment of HIV-1 infection in adults who are virologically suppressed. Elsewhere around the world: Avacta Group – U.K. It is a division of Gamma Biosciences.

The Pharma Data
OCTOBER 26, 2020
Following the unauthorized download of all abstracts on the SITC website, Transgene is communicating the content of the late-breaking poster abstract that will be presented at the SITC 35th Anniversary Annual Meeting (SITC 2020), to be held virtually November 9-14, 2020. Key findings of the trial: .

The Pharma Data
NOVEMBER 5, 2020
In addition to his executive role with The Van Kampen Group, he consults with Altimmune on the development of new vaccines and is a member of the Board of Directors for TNG Pharma which is developing a vaccine for cattle against the toxic effects of the saliva of the horn fly. Van Kampen has been included in Marquis Who’s Who.
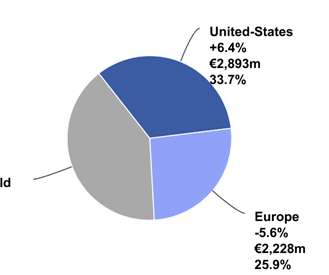
The Pharma Data
APRIL 27, 2021
at CER driven by growth drivers Dupixent ® and Vaccines. Vaccines up 5.3%, driven by PPH franchise and demand for influenza vaccines in southern hemisphere. Vaccines up 5.3%, driven by PPH franchise and demand for influenza vaccines in southern hemisphere. Vaccines delivered growth in its core segments.

pharmaphorum
NOVEMBER 24, 2021
Norwegian biotech Vaccibody has changed its name to Nykode Therapeutics, and celebrated its new identity with a sizeable deal with Regeneron to develop vaccines for cancer and infectious diseases. The US biotech will cover R&D costs as well as development, regulatory, manufacturing and sales for projects that progress.

Roots Analysis
AUGUST 23, 2024
Vaccinations in the past have always been either weak or inactive forms of a pathogen which was given to the body so that our immune system is trained to recognize that given pathogen. There are several advantages to approaching vaccination this way. Ribosomes are cellular machines that read mRNA sequences and produce proteins.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content