Lilly builds case for weekly insulin shot
Bio Pharma Dive
SEPTEMBER 5, 2024
New data show Lilly’s longer-lasting insulin matched daily shots in controlling blood sugar, adding to positive findings the company disclosed in May.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
 Insulin Related Topics
Insulin Related Topics 
Bio Pharma Dive
SEPTEMBER 5, 2024
New data show Lilly’s longer-lasting insulin matched daily shots in controlling blood sugar, adding to positive findings the company disclosed in May.

Bio Pharma Dive
MAY 16, 2024
The results, which showed Lilly’s drug was as effective at controlling blood sugar as standard injections, come as Novo Nordisk seeks approval for a competing once-weekly insulin.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
MAY 24, 2024
The US FDA's EMDAC is set to hold a meeting to assess the benefit-risk profile of Novo Nordisk's insulin icodec, to treat type 1 diabetes.

Bio Pharma Dive
JULY 11, 2024
The FDA’s decision to turn back the drugmaker’s new insulin product narrows Novo’s lead in the U.S. over Eli Lilly, which has a similar diabetes treatment in late-stage testing.

Pharmaceutical Technology
MARCH 8, 2023
Eli Lilly has announced that it will reduce the price of its most commonly used insulin, Humalog, in the US, by 70% and cap the out-of-pocket cost for those on commercial insurance at $35. This is the second time Lilly has significantly reduced the cost of its insulin, as in 2019 the company reduced the list price of Humalog by 50%.

Bio Pharma Dive
MARCH 1, 2023
The pharma company, along with its diabetes drug rivals Novo Nordisk and Sanofi, has long been under pressure over the high cost of insulin — scrutiny that has recently ramped up in the U.S.

World of DTC Marketing
MARCH 31, 2022
The House is preparing to vote on a $35 monthly insulin cap later, but there is stern opposition to the plan, which could cost billions over ten years. With the money it costs us, the Government could encourage a private company to build a manufacturing facility for insulin while offering tax incentives. Los Angeles Times.

Pharmaceutical Technology
AUGUST 2, 2024
Sanofi has announced a significant investment of €1.3bn ($1.4bn) to expand its insulin production capabilities in Frankfurt, Germany.

Fierce Pharma
NOVEMBER 14, 2024
As sales of Novo Nordisk’s GLP-1 drugs for diabetes and obesity continue to soar, the Danish drugmaker is once again adjusting its insulin production priorities. Citing 'capacity constraints,' Novo Nordisk plans to transition its human insulin—which is currently sold in pens and vials—to vials only.

Pharmaceutical Technology
SEPTEMBER 10, 2024
Data from the two Phase III trials showed that efsitora induced HbA1C reductions similar to daily insulin in both type 1 and 2 diabetes.

Bio Pharma Dive
JULY 11, 2022
Gavin Newsom announced a $100 million budget to create a production facility and to develop affordable insulin products, saying the medicines’ high cost “epitomizes market failures.”

Pharmaceutical Technology
SEPTEMBER 10, 2024
Data from the two Phase III trials showed that efsitora induced HbA1C reductions similar to daily insulin in both type 1 and 2 diabetes.
Pharmaceutical Technology
APRIL 18, 2024
At the ongoing Diabetes UK conference, experts highlighted the potential benefits and concerns surrounding weekly insulin candidates.
Bio Pharma Dive
MARCH 3, 2022
The nonprofit group announced ambitious plans to begin offering insulin at prices no higher than $30 per vial by early 2024.
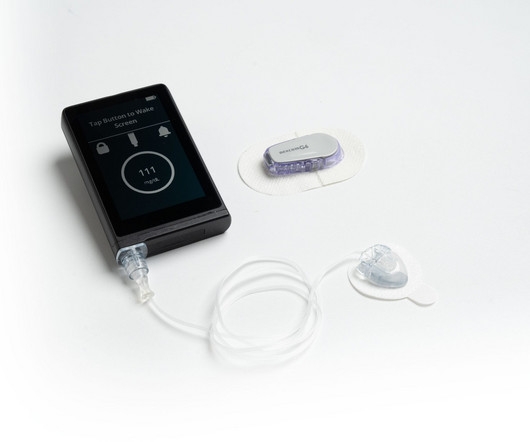
Pharmaceutical Technology
MAY 26, 2023
Chronic disease tech company Convatec Group has partnered with Beta Bionics to manufacture the insulin delivery system iLet Bionic Pancrease. According to Beta Bionics, this is the only first and only automated insulin delivery system that carries 100% of doses and does not require carb counting.

Pharmaceutical Technology
OCTOBER 12, 2022
Novo Nordisk has recently completed its six-part ONWARDS Phase III trial, as ONWARDS 5 reached its primary endpoint with Icodec demonstrating non-inferiority in reducing hemoglobin A1C (HbA1c) in patients with type 2 diabetes (T2D) at week 52 in comparison to once-daily basal insulin analogs. Patients had an overall baseline HbA1c of 8.9%

Pharmaceutical Technology
MARCH 15, 2023
Novo Nordisk has announced plans to reduce the US wholesale acquisition cost (WAC), also known as the list price, of several pre-filled insulin pens and vials by up to 75% for people living with type 1 and type 2 diabetes. Novo Nordisk also has various long-standing US affordability offerings for eligible people who are living with diabetes.

Fierce Pharma
APRIL 18, 2024
Novo Nordisk is receiving pushback from a trio of lawmakers over its decision to discontinue its long-acting insulin product Levemir, which was due for a steep discount at the start of the new year | Novo Nordisk is receiving pushback from a trio of lawmakers on its decision to discontinue its long-acting insulin product Levemir, which was due for (..)
Bio Pharma Dive
JANUARY 13, 2023
The state filed suit Thursday against pharmacy benefit managers CVS Caremark, Express Scripts and OptumRx, alleging they worked with drugmakers to drive up the price of insulin.
pharmaphorum
JULY 11, 2024
FDA rejects Novo Nordisk’s once-weekly basal insulin for people with diabetes, which would reduce the number of injections needed
Pharmaceutical Technology
SEPTEMBER 21, 2023
The licence grants Meitheal marketing rights for insulin aspart, lispro, and glargine following FDA approval.

XTalks
OCTOBER 7, 2024
The US Food and Drug Administration (FDA) has issued a Class I label for the recall of Medtronic’s MiniMed insulin pumps. The devices are used to regulate blood glucose levels by delivering precise amounts of insulin based on readings from an integrated continuous glucose monitor (CGM). This can result in hyperglycemia or hypoglycemia.

Fierce Pharma
JULY 10, 2024
While much of the attention on Novo Nordisk these days revolves around the company’s wildly popular GLP-1 drugs Ozempic and Wegovy, insulin remains a critical backbone for the drugmaker. |
XTalks
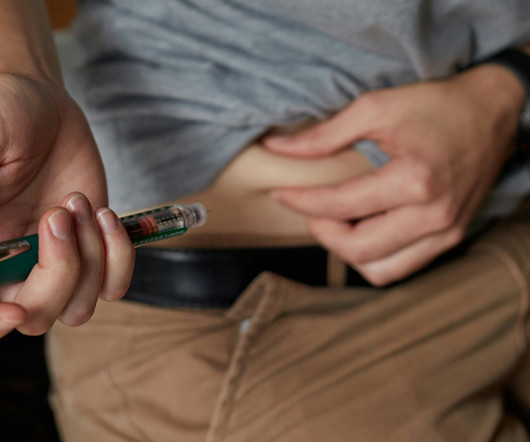
AUGUST 29, 2024
The recent US Food and Drug Administration (FDA) clearance of the Omnipod 5 Automated Insulin Delivery (AID) System for people with type 2 diabetes marks a significant milestone in diabetes care. The Omnipod 5 system offers a personalized approach to insulin delivery, integrating CGM with adaptive insulin administration.
pharmaphorum
JUNE 23, 2024
Three people with type 1 diabetes who received Vertex Pharma’s islet cell therapy VX-880 were able to come off insulin within a year

pharmaphorum
MAY 1, 2024
Polish drug delivery company Biotts says it has demonstrated delivery of insulin across the skin for the first time without the use of an injection system.
Pharmaceutical Technology
JANUARY 10, 2023
In addition, market growth will be driven by dapagliflozin (Farxiga) in the SGLT-2I space, and novel GLP-1/GIP Mounjaro (tirzepatide), biosimilar/interchangeable insulins such as insulin lispro and insulin glargine (Basaglar), and the launch of once-weekly insulins icodec and Icosema, forecast for 2024 launch.

AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 9, 2022
The most effective way for people with diabetes to deliver insulin is also incredibly intrusive. Millions around the world must inject the crucial hormone underneath their skin several times a day to keep their glucose levels in balance.

pharmaphorum
APRIL 13, 2022
The treatment has been around for over a century but insulin has still become the centre of the ongoing discussion over pricing of pharmaceutical products. Ben Hargreaves looks at why insulin’s price is so controversial and whether this could be set to change. Insulin rationing. In the US, approximately 37.3

pharmaphorum
JANUARY 13, 2023
The ‘big three’ insulin producers Novo Nordisk, Eli Lilly, and Sanofi are being sued by the state of California for allegedly working together to set artificially inflated prices for their products. The suit follows legal action over insulin pricing taken by other states, including Arkansas , Kansas, Mississippi, and Minnesota.

NPR Health - Shots
MARCH 19, 2023
Under the $50 million deal, the state is partnering with drugmaker Civica to start making the new generic insulin later this year, Gov. Gavin Newsom said. Image credit: Damian Dovarganes/AP)
Medical Xpress
DECEMBER 12, 2022
WEHI researchers in Melbourne have answered a 100-year-old question in diabetes research: can a molecule different to insulin have the same effect? The findings provide important insights for the future development of an oral insulin pill.

Fierce Pharma
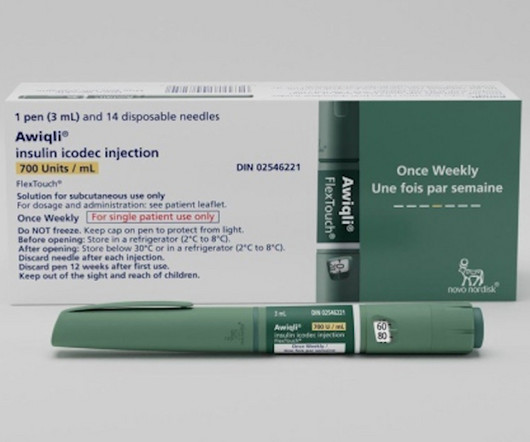
APRIL 29, 2024
For Novo Nordisk to gain approval in the United States for its groundbreaking Awiqli (insulin icodec), it will have to first pass muster with a panel of experts next month,

Bio Pharma Dive
FEBRUARY 8, 2023
In a State of the Union address, Biden threatened to veto any efforts to repeal the legislation and chastised drugmakers for the current costs of insulin.

BioSpace
NOVEMBER 8, 2023
Citing manufacturing concerns and formulary losses, Novo Nordisk will discontinue its long-acting insulin Levemir by the end of next year amid strong pressure from U.S. lawmakers to lower insulin prices.

BioSpace
MAY 22, 2024
In advance of Friday’s Endocrinologic and Metabolic Drugs Advisory Committee meeting, the FDA has raised concerns about hypoglycemia linked to Novo Nordisk’s insulin icodec, according to a briefing document.

Pharmaceutical Technology
MARCH 13, 2024
(Insulin icodec + Semaglutide) is a recombinant peptide and recombinant protein commercialized by Novo Nordisk, with a leading Phase III program in Type 2 Diabetes.

BioSpace
MAY 27, 2024
Flagging a risk of hypoglycemia, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee on Friday did not recommend the approval of Novo Nordisk’s once-weekly insulin icodec for type 1 diabetes.
Medical Xpress
APRIL 21, 2023
Just over a century has passed since the discovery of insulin, a time period during which the therapeutic powers of the hormone have broadened and refined. Insulin is an essential treatment for type 1 diabetes and often for type 2 diabetes, as well. million Americans use insulin, according to the American Diabetes Association.

BioSpace
JULY 11, 2024
The FDA’s Complete Response Letter turned down Novo Nordisk’s application for its once-weekly insulin injection for diabetes mellitus, with “requests” related to the manufacturing process and the type 1 diabetes indication.

Fierce Pharma
MARCH 22, 2024
. | With a positive recommendation from Europe’s Committee for Medicinal Products for Human Use (CHMP), Novo has come a step closer to bringing its revolutionary Awiqli (once weekly basal insulin icodec) to the market. It was one of several opinions handed down by the CHMP.
XTalks
AUGUST 13, 2024
Abbott and Medtronic have struck a global partnership to make diabetes management easier for people who need regular insulin injections. Abbott’s CGM sensors will work with Medtronic’s AID algorithms to automatically adjust insulin levels based on real-time blood sugar readings. billion by 2033.

BioPharma Reporter
SEPTEMBER 12, 2024
New data from the QWINT clinical trial may get Eli Lilly closer to bringing a once-weekly insulin injection to the US, following the FDAâs rejection of Novo Nordiskâs own weekly insulin formulation earlier this year.

pharmaphorum
APRIL 29, 2022
For many patients with type 2 diabetes, having to inject basal insulin every day is a burdensome fact of life, but Novo Nordisk is trying to change that. cases of hypoglycaemia per year with insulin icodec, versus 0.27 In absolute terms, there were 0.73 events for the once-daily drug.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content