mRNA licensing agreements surge 800% amid GSK lawsuits
Pharmaceutical Technology
OCTOBER 18, 2024
An 800% increase in licensing deal values indicates growing confidence in mRNA technology, but some vaccine producers are facing lawsuits.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
 Licensing Related Topics
Licensing Related Topics 
Pharmaceutical Technology
OCTOBER 18, 2024
An 800% increase in licensing deal values indicates growing confidence in mRNA technology, but some vaccine producers are facing lawsuits.

Pharmaceutical Technology
APRIL 18, 2024
Clearmind Medicine has entered into a licensing agreement with Yissum to develop Generation 3.0 psychedelic compounds for mental disorders.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
AUGUST 28, 2023
Specialised Therapeutics has entered into a licensing agreement with Treeway for TW001, designed for treating amyotrophic lateral sclerosis.

Pharmaceutical Technology
FEBRUARY 19, 2024
Almirall has signed a licensing agreement for the acquisition of worldwide rights to Novo Nordisk’s IL21-hindering antibody NN-8828.

Pharmaceutical Technology
MARCH 15, 2024
Anocca has entered a licensing agreement with EmendoBio for the use of the latter’s OMNI-A4 nuclease, a gene editing technology.

Bio Pharma Dive
MAY 12, 2021
The drug, which Biogen had an option to license, is one of two in the biotech's pipeline for a condition that has had few new treatments developed.

pharmaphorum
NOVEMBER 7, 2024
Novo Nordisk has abandoned a drug it licensed from KBP Biosciences last year in a deal valued at up to $1.3
Pharmaceutical Technology
JANUARY 31, 2023
Gene therapy company uniQure has entered into a global licensing agreement with Apic Bio for APB-102 to treat patients with amyotrophic lateral sclerosis (ALS) caused by mutations in superoxide dismutase 1 (SOD1). The post uniQure signs license deal for Apic Bio’s gene therapy for SOD1-ALS appeared first on Pharmaceutical Technology.

Pharmaceutical Technology
DECEMBER 28, 2023
J&J has acquired a global license for South Korean LegoChem’s ADC for $100m upfront along with milestone-based payments and tiered royalties.

Bio Pharma Dive
MAY 16, 2024
The biotech, tentatively named Hercules CM Newco, has rights to three incretin drugs discovered by Jiangsu Hengrui Pharmaceuticals, two of which are in clinical testing.

Pharmaceutical Technology
DECEMBER 8, 2022
The post Kite and Daiichi Sankyo update cell therapy licensing agreement appeared first on Pharmaceutical Technology. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address.

AuroBlog - Aurous Healthcare Clinical Trials blog
JUNE 26, 2024
The Tamil Nadu government has appointed the incumbent controlling authority (CA) of the drugs control administration, M N Sridhar, as state licensing and controlling authority (SLA and CA) for the department of drugs control from June 18 onwards.

Bio Pharma Dive
AUGUST 6, 2024
Through a new deal, Roche has exclusive rights to Sangamo molecules designed to repress the gene that makes “tau,” a protein many scientists view as a main driver of Alzheimer’s.

Bio Pharma Dive
MAY 13, 2024
The deal — worth $100 million up front and potentially billions more later on — gives Takeda an exclusive option to license an Alzheimer’s vaccine and other “active immunotherapies.”

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 24, 2024
In order to better streamline the approval process for manufacturing license to the medical devices units, the Central government is looking at amending the relevant rules in the Medical Device Rules (MDR), 2017 to include various timelines related to grant of manufacturing license to the four classes of medical devices in the country.

Bio Pharma Dive
OCTOBER 12, 2022
The drugmaker’s decision to grab rights to the shot deepens its ties with the COVID-19 vaccine developer and comes weeks before a key data release.
Drug Patent Watch
DECEMBER 12, 2024
Identifying branded drugs with a low likelihood of generic entry has become a crucial strategy for companies looking to expand their product portfolio through in-licensing. In this comprehensive guide, we’ll explore the intricacies of identifying such drugs and leveraging them for successful in-licensing opportunities.

AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 8, 2025
The Central Drugs Standard Control Organisation (CDSCO) and Indian Council of Medical Research (ICMR) have together released the draft standard evaluation protocols for the purpose of issuing license for in-vitro diagnostics (IVDs) under the Medical Devices Rules (MDR), 2017.

AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 13, 2024
The Union Ministry of Ayush will soon amend the Drugs and Cosmetic Rules, 1945 to mandate good manufacturing practices for Homoeopathy drug manufacturing, stipulating procedures for loan licensing in the system of Homoeopathy and specifying timelines for issuance of license, among others.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 23, 2024
The Central Drugs Standard Control Organisation (CDSCO) has requested all stakeholders in the medical devices sector to deposit the requisite fee for retention of license or registration certificate under the Medical Devices Rules (MDR), 2017, well before the stipulated timeline in order to avoid cancellation of the approvals.The drug regulator, in (..)

Pharmaceutical Technology
JANUARY 28, 2025
AB2 Bio has made an option and licensing agreement with Nippon Shinyaku, granting the latter the option to commercialise Tadekinig alfa.

Bio Pharma Dive
MARCH 6, 2023
The Swiss drugmaker's decision is the latest in a string of partnership announcements for the gene therapy maker, which aims to bounce back from past research failures.

Bio Pharma Dive
OCTOBER 29, 2024
The licensing deal with China’s Chimagen Biosciences is the latest example of drugmaker interest in exploring the potential of “T cell engagers” in autoimmune disease.

AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 25, 2024
The Central government’s actions to mandate licensing of all medical devices is posing a challenge to the industry, especially the micro and small enterprises in the sector, says the Surgical Manufacturers and Traders Association (SMTA), the pan India organisation of manufacturers and traders of surgical equipment for healthcare services providers (..)

Bio Pharma Dive
OCTOBER 27, 2021
The deal with the Medicines Patent Pool is meant to increase access in lower-income countries to molnupiravir, an experimental drug that could become the first oral treatment for COVID-19.

Bio Pharma Dive
FEBRUARY 1, 2024
The agreement gives Protagonist an experienced hand in Takeda, which can help the former commercialize its product.

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 20, 2023
Empowering of the Central Drugs Standard Control Organisation (CDSCO) through centralisation of drug licensing may not be the path to improve the quality of drugs manufactured in the country both for domestic and international markets, says SME Pharma Industries Confederation (SPIC), the apex organisation of small and medium pharma industries in the (..)

Pharmaceutical Technology
FEBRUARY 15, 2024
GSK has exercised its option for a licence for Elsie Biotechnologies’ discovery platform to detect and develop new oligonucleotides.

Pharmaceutical Technology
JANUARY 13, 2025
Mediar has entered into a global licensing agreement with Eli Lilly to progress MTX-463 into a Phase II trial for the treatment of IPF.
Bio Pharma Dive
JULY 25, 2022
The Lilly-backed Rona Therapeutics gains rights to a platform for small interfering RNA, along with a slate of preclinical candidates aimed at targets in the liver and other tissues.
Pharmaceutical Technology
MARCH 23, 2023
Biohaven has purchased the exclusive global rights for oral, brain-penetrant dual Tyrosine Kinase 2 (TYK2)/Janus Kinase 1 (JAK1) inhibitor, BHV-8000 (previously TLL-041), which treats immune-mediated brain disorders , from Hangzhou Highlightll Pharmaceutical.
Pharmaceutical Technology
NOVEMBER 20, 2024
Pharmanovia has signed a licensing agreement with Lindis Biotech to commercialise catumaxomab for malignant ascites.

Bio Pharma Dive
NOVEMBER 19, 2024
Elsewhere, former NCI director Ned Sharpless founded a new startup and Novartis licensed another radiopharma drug. The agency turned back Astellas’ attempt to update its drug Izervay’s labeling.
Pharmaceutical Technology
SEPTEMBER 17, 2024
MaxCyte has entered into a license agreement with Kamau Therapeutics, a clinical-stage company focused on stem cell therapy gene correction.

Pharmaceutical Technology
DECEMBER 19, 2023
Sanofi and Innate’s cancer collaboration agreement stretches back to 2016, with two of Sanofi’s NK cell engagers in clinical studies.

Pharmaceutical Technology
SEPTEMBER 13, 2024
Sanofi has signed a licensing agreement with RadioMedix and Orano Med to advance a radioligand therapy, AlphaMedix.

Pharmaceutical Technology
OCTOBER 11, 2024
XtalPi's AI biologics discovery platform, XtalFold, has been licensed to Janssen Biotech for advanced biotherapeutics development.

Pharmaceutical Technology
NOVEMBER 15, 2024
MSD has secured an exclusive worldwide license from LaNova Medicines for developing, manufacturing, and commercialising the latter’s new investigational programmed cell death 1 (PD-1)/vascular endothelial growth factor (VEGF) bispecific antibody, LM-299.

Pharmaceutical Technology
MAY 15, 2024
Enveric Biosciences has entered a non-binding term sheet with MindBio Therapeutics to out-license a class of psilocin prodrugs.
Pharmaceutical Technology
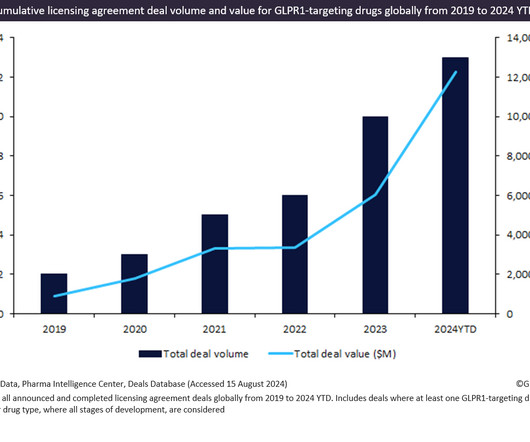
AUGUST 21, 2024
Innovator drugs targeting GLP1R witnessed a 595% increase in total licensing agreement deal value from 2019 to 2024 YTD.

Bio Pharma Dive
JUNE 24, 2021
The pharmaceutical giant is spending $80 million for an exclusive license to an experimental drug developed by Prothena.

Pharmaceutical Technology
FEBRUARY 9, 2024
Nippon will pay $10m upfront and up to $275m in milestone-based payments for the developmental and commercial rights to Vicore’s C21 in Japan.

Fierce Pharma
NOVEMBER 8, 2024
A subcutaneous version of Daiichi Sankyo’s AstraZeneca-partnered blockbuster antibody-drug conjugate (ADC) Enhertu could be in the works from Korea’s Alteogen following a licensing deal worth up to | Alteogen will use its human hyaluronidase hybridization platform to develop a subcutaneous formulation of the blockbuster antibody-drug conjugate.

Bio Pharma Dive
FEBRUARY 10, 2022
The agency criticized Eli Lilly for using a trial run in China to seek approval of an immunotherapy it licensed from Innovent Biologics. Outside advisers agreed in a decisive vote that could have repercussions for several other drugmakers.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content