Novo obesity drug sales lag as manufacturing problems persist
Bio Pharma Dive
NOVEMBER 2, 2022
Manufacturing problems continue to weigh on Novo’s Wegovy launch as competition looms from Eli Lilly’s fast-selling diabetes medicine Mounjaro.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
NOVEMBER 2, 2022
Manufacturing problems continue to weigh on Novo’s Wegovy launch as competition looms from Eli Lilly’s fast-selling diabetes medicine Mounjaro.

Pharmaceutical Technology
OCTOBER 28, 2024
China's virtual monopoly on the supply of many APIs could be dangerous for the security of medicine supply to Western countries.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Bio Pharma Dive
JANUARY 18, 2024
Called Advanced Medicine Partners, the new company will offer specialized cell and gene therapy manufacturing services to biotech and pharma clients.

Bio Pharma Dive
SEPTEMBER 12, 2022
The investment is meant to improve Novartis’ capacity for developing and manufacturing biologic medicines that make up a larger share of its pipeline.

Pharmaceutical Technology
MAY 18, 2023
The European Medicines Agency (EMA) has published recommendations to increase communication and planning efforts in a bid stop the current medicine shortages becoming even worse. The EMA set up a task force in 2016 in light of issues concerning medicine availability and supply chain.

Pharmaceutical Technology
JUNE 7, 2023
The funding will support the new production plant and distribution hub, which is aimed at enhancing access to high-quality medicines in Africa. The manufacturing site will be completed in three phases and will produce anti-bacterial medicines and anti-malarial drugs.

Pharmaceutical Technology
MARCH 10, 2023
On 10 March, the National Health Service Blood and Transplant (NHSBT) opened a new Clinical Biotechnology Centre (CBC) with the aim of improving the UK’s ability to develop and manufacture cell and gene therapies. Personalised medicines will also be developed at the centre.

Pharmaceutical Technology
MARCH 31, 2023
Moderna has finalised an agreement with the government of the Republic of Kenya to establish an mRNA manufacturing facility in the country. The company is also committed to establishing mRNA manufacturing facilities in Australia, Canada, the US and the UK.

Drug Patent Watch
DECEMBER 9, 2024
This intersection is where pharmacognosy meets drug patents, creating a unique landscape that shapes the future of medicine. Let’s embark on a journey to uncover the secrets of nature’s medicine cabinet and the legal frameworks that protect these discoveries.

Bio Pharma Dive
APRIL 22, 2024
The acquisition of a Nexus Pharmaceuticals facility in Wisconsin could help Lilly better meet demand for injectable medicines, like those it makes for diabetes and obesity.

Pharmaceutical Technology
OCTOBER 3, 2024
Eli Lilly has announced a $4.5bn investment to establish the Lilly Medicine Foundry for drug production and manufacturing medicines for clinical trials.

Drug Patent Watch
DECEMBER 10, 2024
The pharmaceutical industry has undergone significant changes over the past decade, with a growing trend towards outsourcing key aspects of research, development, and manufacturing to third-party vendors. The Rise of Integrated CDMOs The global biotechnology and pharmaceutical services outsourcing market size was valued at $70.48
Pharmaceutical Technology
NOVEMBER 21, 2022
RVAC Medicines has signed a master research partnership agreement with the Agency for Science, Technology and Research (A*STAR) for analysing and developing solutions to build messenger ribonucleic acid (mRNA) manufacturing and analytics expertise in Singapore. Topic sponsors are not involved in the creation of editorial content.
Pharmaceutical Technology
JULY 7, 2022
But scientists have struggled to find effective treatments for many of these diseases since the dawn of modern medicine. But there are many challenges it will face along the way, such as how to manufacture these drugs in a reproducible, scalable, cost-effective, and safe way for patients. Securing the supply chain.

Pharmaceutical Technology
FEBRUARY 27, 2025
Eli Lilly has announced plans to expand its domestic medicine production in the US with four new pharmaceutical manufacturing sites.

Pharmaceutical Technology
NOVEMBER 29, 2024
Automated Industrial Robotics have delivered a bespoke Just in Time PACE system to CPI’s new Medicines Manufacturing Innovation Centre.

Bio Pharma Dive
JULY 11, 2022
Gavin Newsom announced a $100 million budget to create a production facility and to develop affordable insulin products, saying the medicines’ high cost “epitomizes market failures.”
Pharmaceutical Technology
AUGUST 30, 2022
International companies investing in the emerging market of Brazilian pharmaceutical manufacturing will see a higher return on investment than from developed market equivalents, if they choose to compete with local manufacturers to supply Brazil’s growing market and the greater South American region.

Bio Pharma Dive
MAY 2, 2022
The regulator cited concerns around single-country trials in turning back Hutchmed's pancreatic cancer treatment, while manufacturing issues held up Junshi and Coherus' throat cancer medicine.

Pharmaceutical Technology
FEBRUARY 3, 2023
The field of genomic medicine has reached a true turning point. In June 2022, the European Medicines Agency approved an adeno-associated viral (AAV) vector-based therapy for adults with Hemophilia A, making the treatment available to an estimated 3,200 eligible patients. [1] CEVEC became part of Cytiva in October 2022.

Bio Pharma Dive
JANUARY 6, 2022
Merck KGaA is buying Indianapolis-based Exelead, a specialist in the lipid nanoparticles used to ferry mRNA into the body, for $780 million in cash.

Bio Pharma Dive
APRIL 2, 2025
Artis BioSolutions joins a host of startups trying to improve development and manufacturing capacity for cutting-edge gene and cell therapies.

Pharmaceutical Technology
OCTOBER 4, 2024
in funding for expanding critical medicine components manufacturing. National Resilience’s subsidiary Resilience Government Services has received around $17.5m

Pharma Times
OCTOBER 17, 2024
The two-year programme will use virtual reality technology to teach essential skills

Bio Pharma Dive
AUGUST 5, 2024
For years, many manufacturers have assumed that pre-market physician education was not strictly necessary unless their brand was the first to market or had a novel mechanism of action. With the advent of precision medicine, however, ongoing disease state education has become increasingly critical.
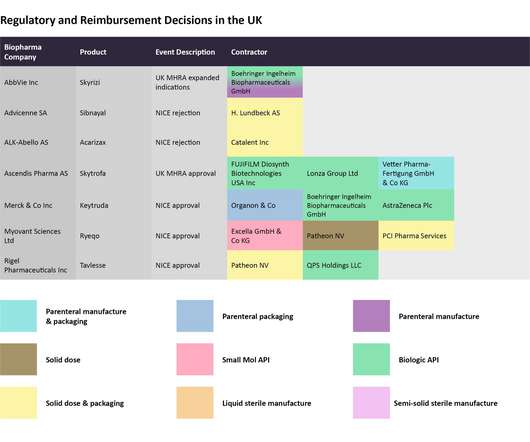
Pharmaceutical Technology
DECEMBER 12, 2022
In this last 2022 edition of the series, which started in June , Pharmaceutical Technology is tracking major trial announcements and decisions by regulators and reimbursement agencies that have occurred since mid-October, as well as their potential impact on manufacturing plans. Iomab-B met the durable complete remission endpoint.

Pharmaceutical Technology
APRIL 18, 2023
Eli Lilly and Company (Lilly) has unveiled plans to invest $1.6bn in its two new manufacturing facilities located within the LEAP Innovation Park in Boone County, Indiana, US. The investment at the LEAP site marks the company’s biggest manufacturing investment at a single location to date.

Drug Patent Watch
JANUARY 23, 2025
With the rise of affordable healthcare initiatives and growing demand for quality medicines, emerging markets present a significant opportunity for generic drug manufacturers to expand their reach and improve access to life-saving treatments. However, generic drug development in emerging markets also presents several challenges.
Pharmaceutical Technology
FEBRUARY 23, 2023
GenScript ProBio has announced a strategic collaboration with RVAC Medicines to manufacture GMP-grade plasmid DNA (pDNA) for the latter’s RVM-V001, an mRNA Covid-19 vaccine candidate. Under the agreement, GenScript ProBio will offer GMP plasmid manufacturing service for the RVM-V001 programme.

Bio Pharma Dive
DECEMBER 16, 2021
The new facility cost nearly $70 million to build, and is part of a major push by the pharma giant to become a leader in genetic medicine manufacturing.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 8, 2024
Last week, the Australian Therapeutic Goods Administration added intravenous (IV) fluids to the growing list of medicines in short supply. The shortage is due to higher-than-expected demand and manufacturing issues. Two particular IV fluids are affected: saline and compound sodium lactate (also called Hartmann’s solution).

pharmaphorum
OCTOBER 25, 2024
GSK has made another investment in its manufacturing network, setting aside $800 million to build two plants at its Marietta site in the US.The programme represents the largest capital spend by the UK drugmaker on production capacity to date and will double the size and capacity of GSK's existing medicines and vaccines facility in Marietta, Pennsylvania, (..)

Pharmaceutical Technology
JULY 17, 2024
Upperton Pharma has received the UK Medicines and Healthcare products Regulatory Agency (MHRA) approval for its facility in Nottingham.

Pharma Mirror
AUGUST 30, 2022
Cytocentric cell manufacturing keeps the critical cell process parameters of temperature, CO2, and O2 at optimum physiologic levels over the entire production process, not just during incubation steps. The post Testing Facility Called “Game Changer” in Regenerative Medicine appeared first on Pharma Mirror Magazine.

Pharmaceutical Technology
FEBRUARY 8, 2023
Genenta Science and AGC Biologics have signed a development and manufacturing service agreement (MSA). Under the deal, AGC Biologics will be responsible for manufacturing the cell therapy lentivirus-based product for the ongoing clinical programmes of Genenta Science.
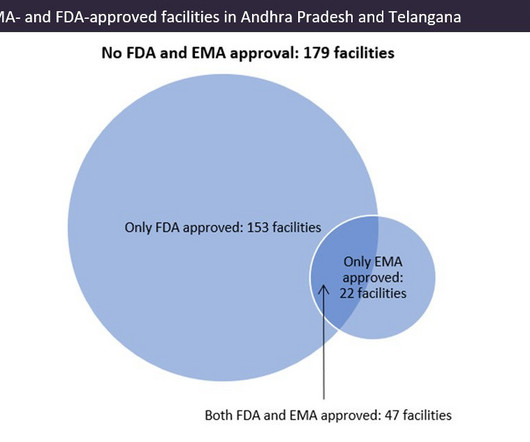
Pharmaceutical Technology
APRIL 4, 2023
Indian pharma manufacturing continues to be the backbone of drug supplies worldwide, and GlobalData analysis suggests US overreliance on the country for generic drug supply. Pharma manufacturing facilities in Andhra Pradesh and Telangana accounted for 22.5% © GlobalData. © GlobalData. There are fewer EMA-approved sites in the region.

Pharmaceutical Technology
DECEMBER 15, 2022
On November 16, 2022, Medicines for Europe (MFE), the generics and biosimilars body, called for EU and national reforms to improve the security of medicine supplies, including tackling the impact of inflation on the security of essential medicine supplies with smart procurement guidelines for medicine.

pharmaphorum
NOVEMBER 21, 2024
Explore how investing in genomic medicine can unlock the true business potential in the manufacturing and therapeutic areas. Understand the promise and reality of this cutting-edge field.

Pharma Mirror
APRIL 18, 2023
Dudley, UK, April 18th 2023: Sterling Pharma Solutions, a global contract development and manufacturing organisation, today announced that it has been granted a Manufacturer’s Authorisation for Investigational Medicinal Products from the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA).

Pharmaceutical Technology
NOVEMBER 30, 2022
However, efficiently manufacturing the drug represents another barrier to cross before realizing the full revenue potential then successfully. Each month, Pharmaceutical Technology takes a look at recent decisions taken by regulatory and reimbursement agencies and identifies the key manufacturing players that can be impacted by them.

STAT News
APRIL 4, 2023
For the first time, Mark Cuban’s Cost Plus Drug Company is selling medicines made by a large drug manufacturer directly to consumers at a greatly reduced price, the latest sign that the billionaire is trying to make good on his vow to disrupt the opaque pharmaceutical supply chain.

Bio Pharma Dive
FEBRUARY 6, 2024
The life sciences investor has backed a number of startups working on technologies for delivering and manufacturing cell and gene medicines.

pharmaphorum
MARCH 15, 2024
Consortium led by Birmingham University unveils a centre of excellence for medicines manufacturing training using VR, to answer a UK skills shortage.

Pharmaceutical Technology
APRIL 25, 2023
Forge Biologics has received a qualified person (QP) declaration to manufacture adeno-associated virus (AAV) gene therapies to support European clinical programmes. The company stated that a European QP has completed an in-depth audit at its manufacturing facility in Columbus, Ohio, US.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content