Overcoming regulatory challenges in sterile manufacturing
Pharmaceutical Technology
MARCH 25, 2025
Evolving regulations in sterile manufacturing requires innovation, market knowledge, and investment. How can drug manufacturers respond?
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
MARCH 25, 2025
Evolving regulations in sterile manufacturing requires innovation, market knowledge, and investment. How can drug manufacturers respond?

Drug Patent Watch
DECEMBER 10, 2024
The pharmaceutical industry has undergone significant changes over the past decade, with a growing trend towards outsourcing key aspects of research, development, and manufacturing to third-party vendors. The Rise of Integrated CDMOs The global biotechnology and pharmaceutical services outsourcing market size was valued at $70.48
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
MAY 2, 2022
The regulator cited concerns around single-country trials in turning back Hutchmed's pancreatic cancer treatment, while manufacturing issues held up Junshi and Coherus' throat cancer medicine.

World of DTC Marketing
FEBRUARY 21, 2022
Pfizer and Merck have chosen to designate a select few generic manufacturers to produce cheaper versions of their drugs through the Medicines Patent Pool (MPP). The post The drug industry continues to dare regulation appeared first on World of DTC Marketing.com. ” More checks from PhRMA to politicians are coming up.
Pharmaceutical Technology
AUGUST 30, 2022
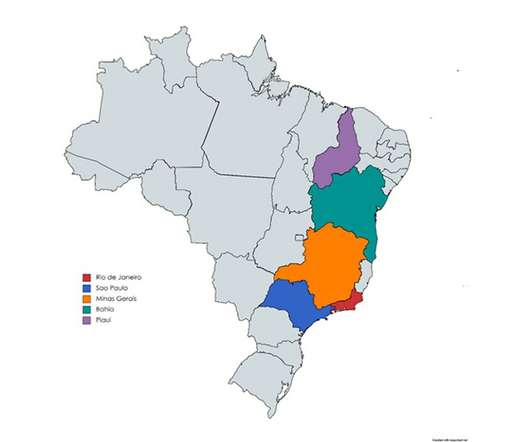
International companies investing in the emerging market of Brazilian pharmaceutical manufacturing will see a higher return on investment than from developed market equivalents, if they choose to compete with local manufacturers to supply Brazil’s growing market and the greater South American region.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 7, 2024
The national drug regulator has withdrawn the powers delegated to the State and Union Territory (UT) Licensing Authorities to issue No Objection Certificates (NOCs) for manufacture of unapproved, banned or new drugs solely for export purpose, asking the industry to file fresh applications with the central authority online from May 15.
Worldwide Clinical Trials
NOVEMBER 12, 2024
The process, from patient coordination through manufacturing and administration, is intricate, time-sensitive, and highly regulated. Understanding Cell Therapy Coordination and manufacture of living IP can be as complex as it is rewarding. Operationalizing these trials requires proactive and flawless management at every stage.
Pharmaceutical Technology
DECEMBER 12, 2022
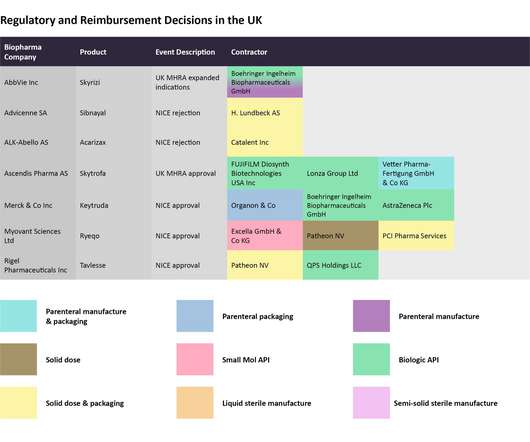
In this last 2022 edition of the series, which started in June , Pharmaceutical Technology is tracking major trial announcements and decisions by regulators and reimbursement agencies that have occurred since mid-October, as well as their potential impact on manufacturing plans. Iomab-B met the durable complete remission endpoint.

Pharma Times
OCTOBER 27, 2023
The new principles will remove the regulatory burden for MLMD manufacturers - News - PharmaTimes

pharmaphorum
JUNE 10, 2022
If you work in pharma, the chances are you’re no stranger to the United States Food and Drug Administration, or FDA, which regulates pharmaceuticals. While the FDA is responsible for regulating both drugs and devices, they’re handled through completely different processes in different parts of the agency.

Bio Pharma Dive
OCTOBER 13, 2020
The biotech won't be able to start human testing of an experimental Huntington's disease treatment until regulators see more manufacturing data.

Pharmaceutical Technology
NOVEMBER 30, 2022
However, efficiently manufacturing the drug represents another barrier to cross before realizing the full revenue potential then successfully. Each month, Pharmaceutical Technology takes a look at recent decisions taken by regulatory and reimbursement agencies and identifies the key manufacturing players that can be impacted by them.

pharmaphorum
MARCH 16, 2021
European regulators questioned the integrity of early batches of Pfizer/BioNTech’s mRNA vaccine, although the matter was resolved before approval, according to information leaked online following a cyberattack. The post Regulators questioned integrity of Pfizer/BioNTech vaccine, leaked files show appeared first on.

Pharmaceutical Technology
SEPTEMBER 8, 2022
Good Therapeutics focuses on the development of PD-1-regulated IL-2 drugs that are based on innovative conditionally active drug technology. With the takeover, Roche will attain rights to a conditionally active, PD-1-regulated IL-2 programme of Good Therapeutics.

BioPharma Reporter
AUGUST 22, 2024
The US regulator has confirmed a delay to the approval of linvoseltamab while a manufacturing issue is resolved with a third-party provider.

pharmaphorum
MARCH 22, 2022
In this article, Ben Hargreaves asks whether there is particular potential for the technologies to revolutionise staff training and reduce costs in pharma manufacturing. The importance of manufacturing. the streamlining of pharma manufacturing in the 21st century. Taking manufacturing to the next level.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 3, 2024
The Central Drugs Standard Control Organisation (CDSCO) has released a list of 59 drug samples declared as Not of Standard Quality (NSQ) during the month of August, with samples of drugs labelled as manufactured by some of the major companies failing the quality test. The State drug regulators have reported 11 NSQs to the CDSCO […]

STAT News
JANUARY 9, 2023
Cough medicine tainted with ethylene glycol that killed at least 19 children in Uzbekistan in late December 2022 has once again revealed lax oversight and regulation of pharmaceutical companies based in India. That preventable tragedy, which involves products made by Marion Biotech, based in Noida, India, echoes earlier cases.

Fierce Pharma
JULY 15, 2024
It's no secret that cell and gene therapies have faced manufacturing hurdles as the advanced medicines have become increasingly popular in recent years. | It's no secret that cell and gene therapies have faced manufacturing hurdles as the advanced medicines have become increasingly popular in recent years.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 29, 2024
The Central Drugs Standard Control Organisation (CDSCO) has released a list of 70 drug samples declared as Not of Standard Quality (NSQ), with samples of drugs labelled as manufactured by some of the major companies failing the quality test.

Drug Patent Watch
DECEMBER 10, 2024
The pharmaceutical and biotechnology industries are heavily regulated, and navigating these regulations can be a significant challenge for companies, especially those with limited experience. Contract Development and Manufacturing Organizations (CDMOs) play a crucial role in helping companies overcome these regulatory hurdles.

AuroBlog - Aurous Healthcare Clinical Trials blog
DECEMBER 12, 2022
The Subject Expert Committee (SEC) for Reproductive and Urology, which advices the nation’s drug regulator on clinical studies and approvals, has recommended approval for the State-run HLL Lifecare Ltd to manufacture and market graphene condom, the next generation thinner; and stronger condom that is expected to improve the acceptability of the (..)

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 16, 2023
The Central Drugs Standard Control Organisation (CDSCO) has approved two more Medical Device Testing Laboratories (MDTL) to carry out tests or evaluation of a medical device on behalf of the manufacturers under the provisions of the Medical Devices Rules (MDR), 2017.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 24, 2022
The Central Drugs Standard Control Organisation (CDSCO) has approved four more Medical Device Testing Laboratories (MDTL) to carry out tests or evaluation of a medical device on behalf of the manufacturers under the provisions of the Medical Devices Rules, 2017.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 11, 2023
The Delhi High Court has granted ten days’ time to the Government of India and the nation’s drug regulator to file a counter affidavit on the petitions filed by almost 28 pharma companies against the order prohibiting manufacturing, distribution and sale of 14 FDCs licensed prior to the year 1988, in the beginning of June. […]

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 12, 2023
The Subject Expert Committee (SEC), which advises the drug regulator on the approval of drugs and clinical trials of antimicrobial and antiviral drugs, has recommended waiver of phase III clinical trial on Emcure Pharmaceuticals’ application for manufacturing and marketing of HIV drug doravirine 100 mg and its bulk drug in the country.Merck Sharp and (..)
Pharmaceutical Technology
APRIL 14, 2023
The US Food and Drug Administration (FDA) has rejected Eli Lilly’s biologic licence application (BLA) for the ulcerative colitis (UC) drug mirikizumab over manufacturing concerns. The regulator has issued a complete response letter. No concerns related to the clinical data package, safety or the medicine label.

Drug Patent Watch
OCTOBER 24, 2024
With the majority of generic drugs manufactured overseas, ensuring compliance with regulatory standards is crucial. This article delves into the complexities of generic drug regulation, highlighting the challenges faced by the U.S. Food and Drug Administration… Source

Pharmaceutical Technology
OCTOBER 26, 2022
Nano-based delivery systems are on the rise, as they enable manufacturers to deliver therapeutic agents to specific targeted tissue in a more controlled manner. Regulations and guidelines for nanopharmaceuticals are still relatively in their infancy, including for cleaning processes and the prevention of cross-contamination.

XTalks
JULY 30, 2024
By focusing on these proactive measures, EU regulations aim to prevent Salmonella contamination before poultry products reach consumers. The contrasting approaches to Salmonella control in the US and EU highlight the importance of evaluating food safety regulations.

Bio Pharma Dive
SEPTEMBER 20, 2023
The drug regulator has twice rejected Alvotech’s biosimilar due to manufacturing issues with a plant in Europe.

AuroBlog - Aurous Healthcare Clinical Trials blog
JUNE 11, 2023
The drug regulator, in a drug alert issued, said that a batch of Typbar, manufactured by Bharat Biotech International Ltd in Genome Valley in Hyderabad, Telangana, failed […]

STAT News
OCTOBER 25, 2022
The antitrust regulator in Spain has fined Merck approximately $39 million for preventing a competitor from marketing a generic version of a hormonal contraceptive, the latest effort by a European government to crack down on anti-competitive practices in the pharmaceutical industry. Continue to STAT+ to read the full story…

BioPharma Reporter
OCTOBER 5, 2023
Sharp, a commercial pharma packaging and clinical trial supply services firm, has acquired Berkshire Sterile Manufacturing (BSM), a Massachusetts-based fill finish contract development and manufacturing organization (CDMO).

BioSpace
OCTOBER 1, 2023
Eli Lilly’s Biologic License Application for its monoclonal antibody lebrikizumab was denied by the regulator after issues were found at a third-party contract manufacturing organization.

Pharmaceutical Technology
APRIL 21, 2023
While the FDA sent Lilly a complete response letter detailing issues pertaining to the proposed manufacturing of mirikizumab, the regulator raised no concerns about the clinical data package or label for the medicine. However, that will depend on how quickly Lilly can address the regulator’s manufacturing concerns.

XTalks
MAY 3, 2024
Challenges and Need for Vigilance: Despite explicit cooking instructions on labels, the persistence of Salmonella -related illnesses underscores the necessity for strict regulations. For example, last month, Trader Joe’s recalled specific fresh basil packages from 29 states following a Salmonella outbreak that affected 12 individuals.

Fierce Pharma
SEPTEMBER 14, 2023
Efforts from regulators and manufacturers have brought the U.S. While there's certainly more work to do, the U.S. supply of cisplatin back to nearly 100% of pre-shortage levels, according to the White House. The moves are “greatly alleviating the shortages of carboplatin,” too, the White House said.

BioPharma Reporter
APRIL 18, 2024
When imagining a pharmaceutical manufacturing facility, many picture it in a vast rural setting, offering ample space for large-scale operations.

Pharmaceutical Technology
JULY 25, 2022
With decreasing margins on the horizon, pharma manufacturers have long shown an interest in specialty generics, which is only expected to rise in the future. Despite this, specialty generics are expected to be the domain of a handful of companies with the necessary manufacturing capabilities and legal backing needed for entering the market.

Pharmaceutical Technology
NOVEMBER 22, 2024
The UK’s proposed regulations would provide the legal framework for POC manufacturing of cell therapies, including quality assurance.

BioPharma Reporter
JANUARY 10, 2023
Catalent is to support the manufacture of Sarepta Therapeutics' gene therapy candidate for the treatment of Duchenne muscular dystrophy (DMD).

Pharmaceutical Technology
SEPTEMBER 4, 2024
Despite the best efforts of pharmaceutical manufacturers and regulators to improve the security and traceability of drug products, counterfeits continue…

XTalks
JUNE 12, 2023
Bristol Myers Squibb (BMS), a multinational pharmaceutical company based in New York City, has announced that its new state-of-the-art cell therapy manufacturing facility in Devens, Massachusetts, has received approval for commercial production from the US Food and Drug Administration (FDA).
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content