Pfizer to pull sickle cell drug from market, shut down trials
Bio Pharma Dive
SEPTEMBER 26, 2024
The withdrawal comes as trial results indicate safety concerns with the drug, Oxbryta, which was first approved in the U.S.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
SEPTEMBER 26, 2024
The withdrawal comes as trial results indicate safety concerns with the drug, Oxbryta, which was first approved in the U.S.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

XTalks
JANUARY 16, 2025
BlueRock Therapeutics, a subsidiary of Bayer AG, is making waves in the treatment of Parkinsons disease by advancing its investigational cell therapy, bemdaneprocel, to a Phase III clinical trial. The clinical advancement follows a thorough review of Phase I trial data under the FDAs Regenerative Medicine Advanced Therapy (RMAT) designation.

Pharmaceutical Technology
JULY 6, 2022
Talks of a bear market in the biotech sector do not seem to pass, with news of companies falling into administration and layoffs becoming a recurrence. Just last week, trading for 4D Pharma , a British biotech, was suspended on the London Stock Exchange’s Alternative Investment Market , and the company will be delisted from NASDAQ on July 7.
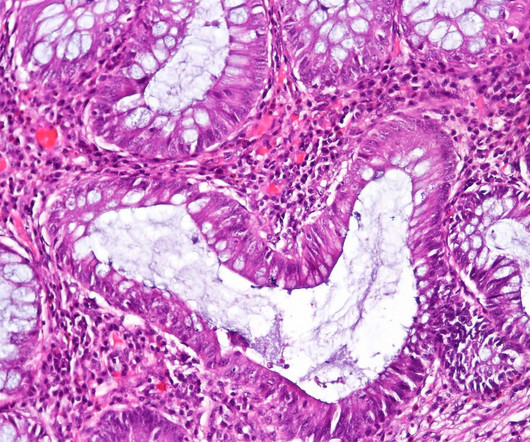
Pharmaceutical Technology
APRIL 3, 2023
The European Commission (EC) has granted marketing authorisation for Sandoz’s biosimilar Hyrimoz (adalimumab) citrate-free high-concentration formulation (HCF). With eight marketed biosimilars, Sandoz is offering the broadest biosimilar portfolio and is the leading biosimilars company in Europe, with more than two decades of experience.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 3, 2023
Pharmaceutical giant Eli Lilly said Thursday it will seek approval to add its diabetes drug to the buzzy US weight loss market after achieving promising results in a new clinical trial. Tirzepatide, currently approved to treat type 2 diabetes under the name Mounjaro, is taken once a week as an injection. Eli Lilly said its […]
World of DTC Marketing
APRIL 20, 2022
We just finished talking to over 200 hundred HCPs about pharma marketing and salespeople to measure any differences since before the pandemic. Attitudes are indeed changing, but one need remains clear: access to data obtained during clinical and ongoing trials. Will they transform their HCP marketing or stick to dated models?

Drug Patent Watch
JANUARY 23, 2025
Designing the Future of Biosimilars: Strategies for Success As the biosimilar market continues to grow, pharmaceutical companies are facing increasing pressure to develop high-quality, cost-effective treatments that meet the evolving needs of patients and healthcare systems. What are your thoughts on the future of biosimilars?

XTalks
DECEMBER 10, 2024
The surge in MASH cases stresses the need for early diagnosis, timely intervention and precise, reliable methodologies in clinical trials to evaluate new therapies effectively. Nicola Owen, PhD, MBBS, Medical Directorthey shared insights into the operational and medical strategies that support the design and conduct of MASH clinical trials.

Pharmaceutical Technology
JULY 26, 2022
According to GlobalData’s recent Niemann-Pick Type C (NPC): Opportunity Analysis and Forecast to 2031 report, the NPC market is expected to see significant growth during 2021–31. This sales growth will be in line with a steadily increasing disease prevalence and the entrance of novel agents into the market.

Bio Pharma Dive
DECEMBER 4, 2024
Results from the head-to-head study showed treatment with Zepbound resulted in 47% greater relative weight loss than Wegovy, a finding that could advantage Lilly in the companies’ market competition.

Bio Pharma Dive
JULY 9, 2021
Zynteglo sales have been on hold since February, when a patient in a clinical trial of another, related Bluebird medicine developed leukemia.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 14, 2024
The Subject Expert Committee (SEC), which advises the national drug regulator on matters related to approval of new drugs and medical devices and clinical trials, has recommended grant of market authorisation for Telangana-based MSN Laboratories for sleep disorder drug Pitolisant tablets.

Pharmaceutical Technology
APRIL 13, 2023
Takeda’s announcement underlines the risk associated with gene therapy R&D at the preclinical stage and the fact that many current AAV programs are unlikely to reach late-stage trials.
Worldwide Clinical Trials
NOVEMBER 12, 2024
Despite their exciting potential, the smooth operation of cell therapy development trials requires extraordinary orchestration, perfectly aligning the product and patient journeys. While clinical supply is essential to any successful trial, autologous cell therapy trials occupy the far end of the spectrum regarding risk tolerance.

Pharmaceutical Technology
JULY 29, 2022
According to GlobalData’s Epidemiology and Market Size database, epidemiologists estimate there are more than 21 million prevalent cases within the tracked 16 countries. Last year saw the most with 167 trials. Cerevel Therapeutics has two Phase II/III trials and three Phase III trials planned.

World of DTC Marketing
FEBRUARY 15, 2022
Biotechs need to think like marketers to cut through the noise and attract investors. CEOs have to think like marketers to cut through the clutter with all the biotech startups out there. 2wo: Clinical trial data releases need to coincide with major medical conferences.

Pharmaceutical Technology
JUNE 15, 2023
Eloxx has revealed its lead candidate ELX-02 improved predicted forced expiratory volume (ppFEV1) in patients with Class 1 cystic fibrosis (CF) in a new analysis of a Phase II trial that missed its efficacy endpoints. The market responded warmly to the reanalysis. The regimen in the trial was a one-week monotherapy period (1.5

Bio Pharma Dive
MARCH 23, 2023
The two companies gained billions of dollars in market value after their antibody drug succeeded against a disease that’s been difficult to treat with biologic medicines.

Bio Pharma Dive
NOVEMBER 27, 2023
market because of negative data, new study results might crack open the door to a relaunch. One year after the U.K. drugmaker withdrew the multiple myeloma drug from the U.S.

Bio Pharma Dive
APRIL 18, 2022
Early analysis of a key clinical trial showed a potentially increased risk of death in cancer patients who received a combination treatment that included TG Therapeutics' approved medicine, Ukoniq.
Worldwide Clinical Trials
APRIL 3, 2024
Are you aware of the challenges you must address for a successful radiopharmaceutical trial? Enhancing Patient Participation in Radiopharmaceutical Trials Patient recruitment is a critical yet challenging part of radiopharmaceutical trials.

FDA Law Blog
JANUARY 30, 2025
However, as we note in that post, the design, timing of initiation, and timely conduct of confirmatory trials are also important considerations in FDAs determination of whether accelerated approval is appropriate. This blog post focuses on interpreting these new authorities with respect to timely conduct of confirmatory trials.

Bio Pharma Dive
AUGUST 9, 2022
The experimental vaccine is the most advanced candidate for the tick-borne disease and, if successful, would be the first to reach the market since withdrawal of GSK’s Lymerix.

Worldwide Clinical Trials
AUGUST 23, 2023
Patients are the backbone of clinical trials, playing an essential role in the drug development process. This engagement is often less understood and is underutilized by sponsors, meaning a significant element of the trial and drug experience is missed during sponsor engagement with the FDA.

Fierce Pharma
DECEMBER 3, 2024
Over the last two-plus years, as Eli Lilly and Novo Nordisk have battled for their share of the massive weight loss market and patients wondered which company’s products were more effective, there | Eli Lilly revealed results from a study that showed its obesity drug Zepbound was 47% more effective than Novo’s Wegovy in helping patients lose weight. (..)

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 6, 2023
The expert committee which advises the drug regulator on clinical trials and drug approvals has recommended for grant of permission for import and marketing of Swiss multinational pharma major Roche’s ophthalmic drug faricimab intravitreal injection in India, with waiver of local phase III clinical trial subject to conditions.

Pharmaceutical Technology
MAY 22, 2023
The European Medicines Agency (EMA) has accepted and verified Sobi’s marketing authorisation application for a new factor VIII (FVIII), efanesoctocog alfa, to treat haemophilia A patients of all ages. In February 2023, the US Food and Drug Administration approved efanesoctocog alfa as ALTUVIIIO.

XTalks
DECEMBER 19, 2024
Roches Alecensa is the current ALK inhibitor market leader. It entered the first-line ALK treatment landscape seven years ago following the Phase III ALEX trial. The trial demonstrated that Alecensa reduced the risk of disease progression or death by 47 percent compared to Xalkori.

Bio Pharma Dive
OCTOBER 10, 2024
Data readouts over the next six months could set expectations for how the highly lucrative market for weight loss therapies will look in the future.

Bio Pharma Dive
JUNE 10, 2024
Positive results from the trial could position Moderna to bring the two-in-one vaccine to market in 2025.

Drug Patent Watch
FEBRUARY 18, 2025
Unlocking the Potential of Biosimilars: Navigating Regulatory Considerations for Clinical Efficacy Trials As the pharmaceutical industry continues to evolve, biosimilars have emerged as a game-changer in the fight against high healthcare costs. Should the trial compare the biosimilar to the reference product, or to a placebo?

Pharmaceutical Technology
APRIL 27, 2023
The European Commission’s (EC) long-anticipated pharma reform plans in the European Union have finally been unveiled , indicating a focus on improving access to medicines across the bloc while cutting down on market exclusivity. Along with the patent-related changes, the EC is seeking to ease some of the regulatory steps.

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 12, 2023
The Subject Expert Committee (SEC), which advises the drug regulator on the approval of drugs and clinical trials of antimicrobial and antiviral drugs, has recommended waiver of phase III clinical trial on Emcure Pharmaceuticals’ application for manufacturing and marketing of HIV drug doravirine 100 mg and its bulk drug in the country.Merck Sharp and (..)
Pharmaceutical Technology
FEBRUARY 8, 2023
On February 7, at a town hall organised to discuss clinical trial designs for gene therapies, FDA experts pushed pharma players to look for ways to establish clinical effectiveness despite the challenges in recruiting patients with rare diseases. GlobalData is the parent company of Pharmaceutical Technology.

Bio Pharma Dive
AUGUST 17, 2022
Trial results position the company to seek approval for indolent systemic mastocytosis, which executives have painted as a “multibillion dollar” market opportunity. Even so, shares fell by double digits in trading Wednesday.

Bio Pharma Dive
OCTOBER 6, 2022
A new fast track designation allows Lilly to begin the process of seeking approval of tirzepatide for obesity, though the drug will need to succeed in a second trial to get to market.
Bio Pharma Dive
DECEMBER 14, 2021
Fitusiran, which Sanofi licensed from Alnylam, could finally get to market after trial delays and safety concerns slowed its progress. But gene therapies and other new medicines might provide competition.

Bio Pharma Dive
FEBRUARY 24, 2025
Early-phase clinical trials, including phase 1 and some phase 2a studies, serve as a pivotal step for biotech companies, laying the foundation for a drug candidate’s journey to market.

AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 2, 2023
The Subject Expert Committee (SEC) that advises the drug regulator of the country has recommended waiver of local phase III and IV clinical trials in India for Sanofi Healthcare India’s orphan drug to treat the Pompe disease. The Committee recommended grant of permission to import and market the drug without these stages of studies.
Pharmaceutical Technology
AUGUST 30, 2022
International companies investing in the emerging market of Brazilian pharmaceutical manufacturing will see a higher return on investment than from developed market equivalents, if they choose to compete with local manufacturers to supply Brazil’s growing market and the greater South American region.

Pharmaceutical Technology
AUGUST 18, 2023
Depending on Phase III results, survodutide could soon enter the weight loss market, which is expected to reach $37.1bn by 2031.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 23, 2023
The Central drug regulator has issued alert on suspected falsified versions of Glucagon-like peptide 1 receptor agonist (GLP-1-RA) products, an anti-diabetes management drug, in the market. GLP-1-RA […]

Bio Pharma Dive
SEPTEMBER 27, 2021
Phase 3 results showed Merck's drug improved overall survival versus placebo, which should lessen pressure for a market withdrawal in the indication.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?



Let's personalize your content