BMS and Prime ink potential $3.5bn deal to develop T cell therapies
Pharmaceutical Technology
OCTOBER 1, 2024
As part of the deal, which could be worth $3.5bn, BMS will gain developmental support and therapeutic reagents from Prime Medicine.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharmaceutical Technology
OCTOBER 1, 2024
As part of the deal, which could be worth $3.5bn, BMS will gain developmental support and therapeutic reagents from Prime Medicine.

Scienmag
OCTOBER 2, 2020
Addresses major testing need in developing world; also in US, where reagent supplies are again dwindling Credit: Brian Jenkins A major roadblock to large scale testing for coronavirus infection in the developing world is a shortage of key chemicals, or reagents, needed for the test, specifically the ones used to extract the virus’s genetic material, (..)
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Scienmag
MARCH 5, 2021
However, it requires expensive laboratory equipment and global shortages of reagents […]. Evolutionary Anthropology Quantitative real-time polymerase chain reaction (qPCR) is the most widely used diagnostic method to detect RNA viruses such as SARS-CoV-2.

The Pharma Data
MARCH 23, 2022
Roche today announced the donation of additional medicines and diagnostics to Ukraine. Beyond the already communicated 150,000 packs of an antibiotic medicine the new donation includes another 4,600 packs of specialized medicines for the treatment of influenza, rheumatoid arthritis, SMA and various cancers.
Scienmag
OCTOBER 15, 2020
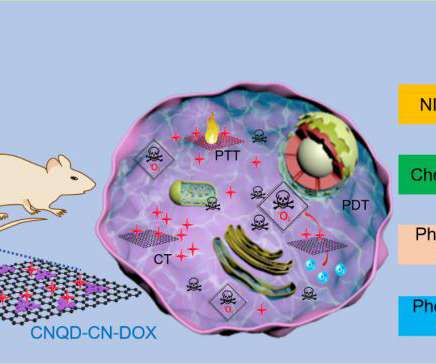
LIN Wenchu from the High Magnetic Field Laboratory of the Hefei Institutes of Physical Science developed a synthesis of metal-free multifunctional therapeutic reagents, called graphitic carbon nitride quantum dots embedded in carbon nanosheets (CNQD-CN), via a one-step hydrothermal treatment. WANG Hui and Prof.

pharmaphorum
JUNE 1, 2021
Pharmaceutical companies are accountable for ensuring that their medicine is safe when it comes into the hands of patients. Specific apps could also alert healthcare providers if vulnerable patients are failing to scan their medicine. This technology can also play an important role in supporting virtual clinical trials.

pharmaphorum
AUGUST 16, 2020
Driven by technological advances and the development of precision medicines, these modernization initiatives are designed to propel laboratory efficiencies into the future, allowing scientists to spend more time on science. Reorder reagents. Notify of spill/cleaning needed. Equipment occupancy notification. Summon a lab runner.

Camargo
MAY 2, 2021
Advances in scientific knowledge and growth in the cell and gene therapy space have led to a new and exciting era of medicine for patients, as well as a new motivation for regulators to provide clear, efficient pathways for product developers. Note: Some medicines are in more than one category.

Pharmaceutical Technology
SEPTEMBER 14, 2022
Emerging technologies such as 3D printing and bioprinting, electrospinning, electrospraying, microfluidics, nanofluidics, microelectromechanical, and bio-electromechanical systems are being utilised to prepare systems for the delivery of personalised medicine, meeting patient’s needs, and developing old drugs with new processes.

Scienmag
MAY 18, 2021
(Boston)–Researchers from Boston University School of Medicine (BUSM) report the formation of human cells containing a green fluorescent protein or GFP (one of the most important proteins in biology and fluorescence imaging) genetically fused with two interferon stimulated genes (ISGs), namely Viperin and ISG15.

The Pharma Data
MARCH 20, 2021
The Challenge: The demands of many labs simultaneously beginning COVID-19 screening programs are leading to a scarcity of resources like pipette tips and reagents. Medicine in Oxford, Biogazelle, a biotechnology company in Ghent, and the Centre For Proteome Research at the University of Liverpool. delays where reagents are in.

FDA Law Blog
JANUARY 4, 2023
As you may know, there are challenges related to developing potency assay(s), and the Alliance for Regenerative Medicine and the American Society of Gene and Cell Therapy recently published a white paper on a workshop held to discuss these challenges. If I use GMP grade reagents, isn’t that sufficient to support their safety?
Pharmaceutical Technology
FEBRUARY 15, 2023
RNA has the potential to underpin breakthrough treatments for a wide variety of diseases, including many cancers, and transform medicine as we know it. Outlook The era of mRNA-based genomic medicines is on the horizon. But the mRNA technology is not yet mature and there are no standardised manufacturing protocols yet.

The Pharma Data
MAY 4, 2021
The New Zealand Medicines and Medical Devices Safety Authority (Medsafe) has banned the trade in unapproved COVID-19 vaccines for a year to prevent the use of vaccines that may not be safe or effective. . TGA warns of unlawful advertising of listed medicines. The public could also import personal supplies of vaccines. CDSCO Notice.

Roots Analysis
FEBRUARY 21, 2022
Advancement in DNA sequencing technologies have resulted in noteworthy developments in various healthcare-related research fields, such as diagnostics and personalized medicine. Moreover, the manual protocols require extensive manipulation, costly reagents and long duration of skilled genomic library production.

The Pharma Data
MARCH 17, 2021
Standardisation enables labs to do more work on fewer instruments, through consolidation of workflows, systems and reagents. 8,9 Additionally, the plastic generated per test result has been reduced by up to 78% due to smaller reagent pack sizes with higher number of tests per pack.
pharmaphorum
FEBRUARY 3, 2021
This can be partly attributed to the reagents and delivery modalities that were available at the time of the trial start in 2016, leading to low editing efficiency at the target loci. The rapid progress and promising outcomes to date make base editing a strong contender in this exciting and fast-paced era of precision medicine.

pharmaphorum
OCTOBER 7, 2020
The company said the problems – which also include other items like reagents and screening kits – have resulted from a switch to a new warehouse, which ironically is intended to make production quicker and more efficient through the use of automated processes.

The Pharma Data
JANUARY 24, 2021
As part of his work with Juno and Kite, Dr. Bond led R&D collaborations with genome editing companies including Editas Medicine and Sangamo Therapeutics. Bond led the development of allogeneic cell therapy platforms leveraging T cells from both donor-derived sources and induced pluripotent stem cells. FRSC at the University of Toronto.

pharmaphorum
JANUARY 25, 2022
The Toronto-based company already counts many of the world’s largest pharma companies among its customers, using its platform for a range of tasks such as improving reagent and antibody selection to help scientists run more successful experiments, drawing on data from published studies and organisations’ internal databases.

Pharmaceutical Technology
FEBRUARY 3, 2023
The field of genomic medicine has reached a true turning point. In June 2022, the European Medicines Agency approved an adeno-associated viral (AAV) vector-based therapy for adults with Hemophilia A, making the treatment available to an estimated 3,200 eligible patients. [1] CEVEC became part of Cytiva in October 2022.

FDA Law Blog
AUGUST 3, 2022
In the LDT’s “toddler years,” when issuing its analyte specific reagent rule in 1997, FDA stated that it would generally exercise enforcement discretion over LDTS. Tests created by labs were important in 1992, but they play a far more vital role in medicine today.
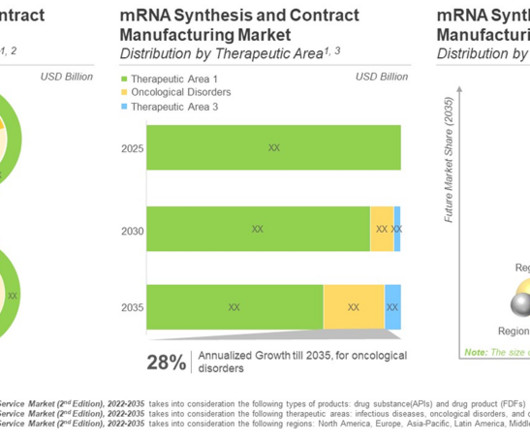
Roots Analysis
AUGUST 16, 2023
mRNA has completely revolutionized the field of medicine and significantly influenced the study and treatment of human diseases. The success of COVID-19 vaccines paved the path for mRNA-based drug products. Safety, efficacy and rapid production of mRNA drives the interest in this domain.

FDA Law Blog
NOVEMBER 1, 2023
The PR first sets out to establish that it has authority to regulate in vitro diagnostic “test systems” as devices, and not just the system’s individual components, such as reagents, instruments, specimen collection devices, and software. The PR states that LDTs are not the “practice of medicine,” with which FDA generally may not interfere.

The Pharma Data
SEPTEMBER 8, 2021
TIB Molbiol is a biotech company that has supplied the global market with reagents for research and medical diagnostics for over 30 years. We are looking forward to contributing to the strong network of Roche Diagnostics.”. About TIB Molbiol. The majority of assays are used to test for infectious diseases.

The Pharma Data
MAY 17, 2021
So asserted Lutz Uharek, a professor of internal medicine and founder and CEO of Xencura, at RAPS Euro Convergence 2021 on 12 May in discussing some of the challenges and opportunities for academic centers in developing cell and gene therapies.

The Pharma Data
DECEMBER 2, 2020
ERS Genomics – Ireland’s ERS Genomics Limited, and Germany’s Vivlion GmbH, announced a non-exclusive license agreement granting Vivlion access to ERS Genomics’ CRISPR/Cas9 patent portfolio, to enhance Vivlion’s gene editing reagents and screening services.

The Pharma Data
MARCH 16, 2022
3, and Delta ([link] About TIB Molbiol TIB Molbiol is a subsidiary of Roche Diagnostics that has supplied the global market with reagents for research and medical diagnostics for over 30 years. These kits can detect and differentiate between Omicron subvariants BA.1, The majority of assays are used to test for infectious diseases.
Advarra
NOVEMBER 29, 2022
However, despite the promise of these therapies, the regulations governing them lag the science, which in turn hinders the clinical translation of these novel medicines. The FDA recently announced a new initiative as part of its NIH Accelerating Medicines Partnership Program. But review issues are not the only problems.
The Pharma Data
MARCH 13, 2021
Hereditary Angioedema (HAE), like so many other rare diseases, is highly complex, and patients, their families and caregivers often undergo years of strain trying to understand their disease, get a definitive diagnosis and gain access to the medicines they need. At Takeda we are a committed champion for the patients we serve. Interactions.

The Pharma Data
OCTOBER 19, 2020
DB ( Becton, Dickinson and Company ) received a CE mark for its BD Multitest 6-Color TBNK Reagent with BD Trucount Tubes for assessing immune function in COVID-19 patients. It allows the test to be used for people without any symptoms or suspicion of COVID-19, supporting widespread population testing. Please read more here. .
The Pharma Data
JULY 11, 2021
Hereditary Angioedema (HAE), like so many other rare diseases, is highly complex, and patients, their families and caregivers often undergo years of strain trying to understand their disease, get a definitive diagnosis and gain access to the medicines they need. At Takeda we are a committed champion for the patients we serve. Interactions.

The Pharma Data
MAY 15, 2023
She is also a board member at BBI Solutions, a UK based diagnostic reagents and a Novo Holdings company. Shamiram holds an AB from Smith College and Doctor of Medicine as well as a Master of Public Health, both from Emory University, U.S.A. About Novartis Novartis is reimagining medicine to improve and extend people’s lives.

XTalks
OCTOBER 19, 2023
This could involve tweaking experimental conditions, changing reagents or redesigning the assay. There is also an increasing focus on personalized and precision medicine, where treatments are tailored to individual patients based on genetic or molecular data, which requires assays to detect and quantify these unique markers.

XTalks
OCTOBER 1, 2020
Prior to starting Fountain Therapeutics in 2018, with co-founders Tom Rando, MD, PhD and Tom Cheung, PhD, Dr. Rodgers was an assistant professor at The Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at the Keck School of Medicine of The University of Southern California (USC).

The Pharma Data
OCTOBER 14, 2020
Prestige BioPharma – Singapore-based Prestige received a positive opinion from the European Medicines Agency Orphan Drug Commission (COMP) for its first-in-class anti-PAUF monoclonal antibody, PBP1510, for the treatment of pancreatic cancer. Talking Medicines – Scotland’s Talking Medicines will join Tech Nation’s Applied AI 2.0

The Pharma Data
SEPTEMBER 7, 2020
The test offers an alternative to lab-developed tests (LDTs) or Analyte Specific Reagent (ASR) combinations, potentially minimising variability and complexity in testing, reducing workload and alleviating risk for laboratories. The cobas BKV Test has robust coverage with a limit of detection of 21.5 IU/mL to 1E+08 IU/mL in EDTA plasma.

The Pharma Data
AUGUST 4, 2020
The test offers an alternative to lab-developed tests (LDTs) or Assay Specific Reagents (ASR) combinations, potentially minimising variability and complexity in testing, reducing workload and alleviating risk for laboratories. The cobas EBV test has robust coverage with a limit of detection of 18.8

XTalks
JANUARY 6, 2025
Whether its the integration of nanotechnology in medicine, the evolution of point-of-care (POC) diagnostics or the transformative impact of CRISPR and regenerative medicine, these biotech trends are pushing scientific boundaries and creating new opportunities for businesses and researchers alike. billion by 2031.

The Pharma Data
DECEMBER 23, 2020
The agreement includes a £500,000 upfront payment to Avacta which gives Astrea the rights to generate and develop Affimer reagents in-house for affinity separation. BioNTech – Germany’s BioNTech and partner Pfizer secured authorization from the European Medicines Agency for its COVID-19 vaccine. It is a division of Gamma Biosciences.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content