GSK and Flagship Pioneering link for new medicines and vaccines
Pharmaceutical Technology
JULY 30, 2024
GSK has announced a strategic collaboration with Flagship Pioneering for the discovery and development of medicines and vaccines.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
JULY 30, 2024
GSK has announced a strategic collaboration with Flagship Pioneering for the discovery and development of medicines and vaccines.

Bio Pharma Dive
JULY 22, 2022
The positive decision comes as governments aim to expand supply and quicken distribution of the vaccine to combat a widening global outbreak. Separately, the EMA backed approvals of 11 other medicines.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
MAY 30, 2023
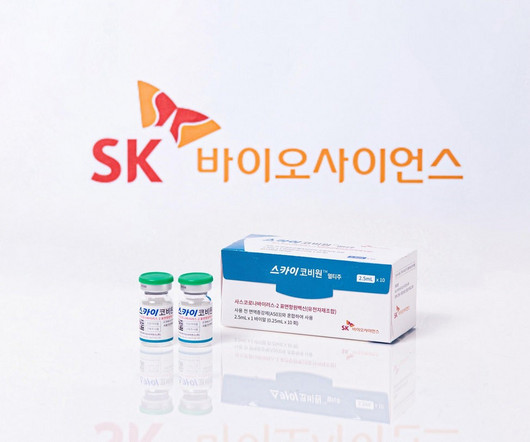
SK Bioscience has received marketing authorisation from the UK’s medicines and healthcare products regulatory agency (MHRA) for its Covid-19 vaccine, SKYCovion. The authorisation allows the distribution of the vaccine in Scotland, Wales and England.
Pharmaceutical Technology
MARCH 24, 2025
The FDA has granted approval for the IND application of Everest Medicines tumour-associated antigen (TAA) vaccine, EVM14.

Bio Pharma Dive
MAY 25, 2022
The pharma has been criticized for not doing enough to make its COVID-19 vaccine and pill available globally. The new initiative commits Pfizer to supplying current and future branded medicines at lower cost to 45 countries.

Pharmaceutical Technology
AUGUST 17, 2022
On August 8, Pfizer and Valneva announced the initiation of a Phase III study with their Lyme disease vaccine , bringing the prospect of an injection to prevent the condition disease one step closer to reality. This means that the vaccine works against multiple serotypes of the disease, she explains. A one-size-fits-all approach.
Pharmaceutical Technology
FEBRUARY 3, 2023
Only a few weeks into the new year, the prospect of getting a successful advanced HIV vaccine shrank after the discontinuation of yet another late-stage trial. On January 18, Janssen, a Johnson & Johnson (J&J) subsidiary, stated that its vaccine was not effective in preventing HIV infections.

Pharmaceutical Technology
OCTOBER 2, 2023
The Physiology and Medicine Prize has gone to two researchers whose work laid the foundation for Pfizer and Moderna’s Covid-19 vaccines.
Pharmaceutical Technology
FEBRUARY 7, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorisation for Takeda ’s dengue virus vaccine candidate, Qdenga (Dengue Tetravalent Vaccine [Live, Attenuated]). The vaccine candidate has been approved for active immunisation against the infection in people from four years of age.

Pharmaceutical Technology
APRIL 14, 2023
Ghana’s Food and Drug Authority (FDA) has approved R21/Matrix-M malaria vaccine in children aged 5 to 36 months, marking the first regulatory clearance for the University of Oxford-developed vaccine in any country in the world. Children between the ages of five and 36 months are at highest risk of death from malaria.
Pharmaceutical Technology
FEBRUARY 23, 2023
GenScript ProBio has announced a strategic collaboration with RVAC Medicines to manufacture GMP-grade plasmid DNA (pDNA) for the latter’s RVM-V001, an mRNA Covid-19 vaccine candidate. GenScript ProBio CEO Dr Brian Min said: “We are delighted to enter into this strategic partnership with RVAC Medicines.

Bio Pharma Dive
MAY 19, 2023
The biotech, best known for its vaccine research, said its clinical trial is the first to report results of a messenger RNA therapeutic designed for intracellular protein replacement.

Pharmaceutical Technology
MARCH 20, 2023
RVAC Medicines has announced a research collaboration with the University of Pennsylvania (Penn) for the discovery and development of mRNA vaccines. The mRNA vaccine candidates will help reduce the chances of autoimmune responses that might lead to allergic conditions or serious autoimmune diseases.
Pharmaceutical Technology
JUNE 20, 2023
The World Health Organisation (WHO) has granted an emergency use listing (EUL) to SK bioscience’s Covid-19 vaccine, SKYCovione. SKYCovione is a self-assembled nanoparticle vaccine and the 12th Covid-19 vaccine to receive a EUL from the regulator.

Bio Pharma Dive
FEBRUARY 2, 2021
The estimate for 2021 sales, which Pfizer splits with partner BioNTech, would make the vaccine one of the highest-selling medicines in the industry.

Pharmaceutical Technology
JUNE 24, 2022
As regulatory agencies gear up for another round of Covid-19 vaccine deliberations centered on emerging variants, Moderna has released data on its booster’s efficacy against Omicron subvariants. The mRNA-1273.214 booster contains the original Spikevax vaccine and a candidate targeting Omicron BA.1 1 variant of concern.

Bio Pharma Dive
JANUARY 11, 2021
Study results could come soon for several coronavirus vaccines, as well as experimental medicines from Amgen, Vertex and Sage Therapeutics.

Pharmaceutical Technology
APRIL 6, 2023
Moderna has announced that its cancer vaccine mRNA-4157/V940 along with Keytruda secured the European Medicines Agency (EMA) Priority Medicines (PRIME) scheme designation for the adjuvant treatment of high-risk stage III/IV melanoma patients after complete resection.

Bio Pharma Dive
JULY 2, 2021
The agency is under pressure to grant full approvals to two coronavirus vaccines and faces important questions on how to handle the next Alzheimer's drugs after Aduhelm. A safety review of several arthritis medicines, meanwhile, is ongoing.
Pharmaceutical Technology
FEBRUARY 21, 2023
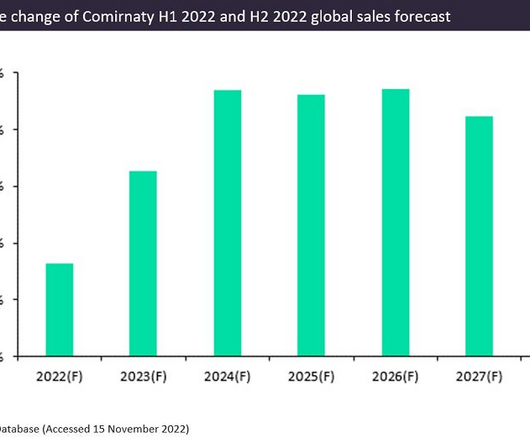
Pfizer and BioNTech’s Covid-19 vaccine, Comirnaty, had a phenomenal year with forecast sales of $37bn in 2022. Comirnaty is the leading prophylactic vaccine for Covid-19 and is expected to generate an additional $2.8bn in sales in 2022 compared to GlobalData’s H1 2022 forecast. Comirnaty is the first globally approved Covid-19 vaccine.
Pharmaceutical Technology
AUGUST 15, 2022
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted conditional authorisation for Moderna ’s Covid-19 booster vaccine, mRNA-1273.214 (Spikevax Bivalent Original/Omicron), for use in adults aged 18 years and above. In addition, no serious safety concerns linked to the vaccine were observed.

Pharmaceutical Technology
MAY 25, 2023
A new vaccine developed by the Serum Institute of India to fight meningococcal disease could help eliminate meningitis across Africa. The results from a trial, published in The New England Journal of Medicine , found the vaccine was associated with a strong immune response and good safety profile. for serogroup W to 20.5

Bio Pharma Dive
APRIL 15, 2021
The decision to end development of a medicine acquired in a $425 million deal last November is another disappointment for Merck's coronavirus effort, which once involved multiple drugs and vaccines.

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 20, 2025
A Parliamentary Panel has recommended implementation of a comprehensive, nationwide and phased Medicines Regulatory Maturity Enhancement Programme on the lines of World Health Organisation’s (WHO) vaccine benchmarking process to ensure a consistently high standard of drug regulation across the country.

Velocity Clinical Research
MARCH 19, 2025
Charles Eger, MD, contributed to new research on a combination vaccine intended to provide protection against both seasonal flu and COVID-19 in a single shot. Full article: [link] The post Charles Eger, MD, Authors New Article in Nature Medicine appeared first on Velocity Clinical Research.

Pharmaceutical Technology
APRIL 28, 2023
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of GSK’s respiratory syncytial virus (RSV) vaccine candidate for use in older adults. This is the first time a vaccine candidate for the potential treatment of adult RSV patients has received a positive opinion.

Pharmaceutical Technology
FEBRUARY 5, 2025
While vaccine sales dropped, GSK raised its revenue guidance for 2031 with speciality medicines expected to drive that growth.

Pharmaceutical Technology
NOVEMBER 6, 2023
GlobalData uncovers the leading innovators in personalized cancer vaccines for the pharmaceutical industry.

Pharmaceutical Technology
APRIL 17, 2023
Health Canada has accepted Pfizer Canada’s new drug submission for a bivalent respiratory syncytial virus (RSV) vaccine for review. The vaccine will be used to prevent lower respiratory tract disease and severe lower respiratory tract disease caused by RSV in adults aged 60 and above.
Pharmaceutical Technology
NOVEMBER 21, 2022
RVAC Medicines has signed a master research partnership agreement with the Agency for Science, Technology and Research (A*STAR) for analysing and developing solutions to build messenger ribonucleic acid (mRNA) manufacturing and analytics expertise in Singapore. Topic sponsors are not involved in the creation of editorial content.
Pharmaceutical Technology
NOVEMBER 11, 2022
The European Commission (EC) has granted approval for Sanofi and GSK ’s monovalent, recombinant-protein-based, adjuvanted Covid-19 vaccine, VidPrevtyn Beta, as a booster in adults aged 18 years and above. It is indicated as a booster in people of this age group who were earlier inoculated with a Covid-19 vaccine.

Pharmaceutical Technology
JUNE 8, 2023
The European Commission (EC) has granted marketing authorisation for GSK’s respiratory syncytial virus (RSV) vaccine, Arexvy, for adults aged 60 years and above. The vaccine is indicated for active immunisation to prevent RSV-related lower respiratory tract disease (LRTD) in older adults.

Pharmaceutical Technology
SEPTEMBER 22, 2022
It could be that you are lucky, and there is already a cancer vaccine on the market that you will get priority for. Alternatively, there are vaccines ready to enter decentralised third-phase clinical trials, so you can enrol quickly.
Pharmaceutical Technology
SEPTEMBER 14, 2022
Novavax and Serum Institute of India (SII) have reported that the former’s Covid-19 vaccine, NVX-CoV2373, has received full product registration from the South African Health Products Regulatory Authority (SAHPRA), with conditions. The vaccine showed efficacy, with an encouraging safety and tolerability profile, in the trials. .

STAT News
NOVEMBER 15, 2022
As the Covid-19 pandemic spread across the world two years ago, one of India’s leading biotech companies was racing to develop a vaccine with crucial backing from the Indian government. Read the rest…

Fierce Pharma
MAY 8, 2024
On Tuesday, the EMA said it had accepted a request from AZ and withdrawn the company’s marketing authorization for its COVID-19 shot Vaxzevria.

pharmaphorum
MARCH 16, 2021
European regulators questioned the integrity of early batches of Pfizer/BioNTech’s mRNA vaccine, although the matter was resolved before approval, according to information leaked online following a cyberattack. As it conducted its analysis of the vaccine in December, the European Medicines Agency’s systems were targeted by unknown hackers.

Pharmaceutical Technology
MAY 30, 2023
Valneva has submitted a regulatory application to Health Canada, seeking marketing approval for its chikungunya vaccine candidate, VLA1553. The vaccine is designed to be administered as a single shot and is intended for individuals aged 18 years and older. Valneva plans to make further regulatory submissions.

Pharmaceutical Technology
JUNE 7, 2023
Covid-19 vaccine compositions should be updated to provide protection against XBB Omicron variant strains in preparation for the autumn vaccination campaign, say EU agencies in a joint 6 June statement. There, organisations determined that the compositions of Covid-19 vaccines need to be updated to reflect the evolution of the virus.

Pharmaceutical Technology
MAY 24, 2023
In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Recombinant viral vector-based medicinal preparations. However, not all innovations are equal and nor do they follow a constant upward trend.

BioSpace
MAY 14, 2021
A study, published recently in the New England Journal of Medicine, demonstrated no obvious safety signals among pregnant persons who received the mRNA vaccines.
Pharmaceutical Technology
APRIL 10, 2023
It will also be used for the treatment of people who are not likely to have an adequate immune response to Covid-19 vaccination or for people who are not recommended for Covid-19 vaccination. Dr Noor Hisham was quoted as saying: “The use of Evusheld was based on clinical guidelines issued by the health ministry.”

Pharma Mirror
SEPTEMBER 11, 2022
Bandung, W Java, Indonesia, PT Bio Farma, the holding company for state-owned pharmaceutical companies in Indonesia, announced a new milestone in the manufacturing of IndoVac, a Covid-19 vaccine brand it has developed since November 2021.

Pharmaceutical Technology
FEBRUARY 1, 2023
RSV researchers at major pharmaceutical companies are currently working to develop new RSV drugs to beat future waves of RSV infection and gain the first RSV vaccine FDA approval. Pharmaceutical companies are pushing to develop drugs and vaccines for RSV with these populations in mind.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content