Phony Diagnoses Hide High Rates of Drugging at Nursing Homes
NY Times
SEPTEMBER 11, 2021
At least 21 percent of nursing home residents are on antipsychotic drugs, a Times investigation found.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

NY Times
SEPTEMBER 11, 2021
At least 21 percent of nursing home residents are on antipsychotic drugs, a Times investigation found.

Scienmag
APRIL 9, 2021
Credit: Indiana University Since 2016, a federal regulation has allowed nurse practitioners and physician assistants to obtain a waiver to prescribe buprenorphine, a medication used to treat opioid use disorder as a medication assisted treatment.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

NY Times
OCTOBER 15, 2021
Diagnoses for the disorder have soared in the past decade, driven in part by a loophole in regulations. A new study shows the impact has been more severe for Black Americans.

STAT News
DECEMBER 23, 2022
These highly regulated facilities are made up of multidisciplinary teams of physicians, nurses, counselors, and social workers who provide comprehensive medication-assisted treatment. Many Americans with opioid use disorder go to one of the nearly 2,000 opioid treatment programs (OTPs) in the U.S. Read the rest…

Pharmaceutical Technology
FEBRUARY 23, 2023
He practices biohacking on the less invasive and low-tech side with intermittent fasting or fasting completely on a regular basis intended to maintain a healthy weight and regulate his blood sugar levels. nurses, doctors, and hospital auxiliary staff) to meet these needs.

Outsourcing Pharma
NOVEMBER 16, 2022
In-home nurse visits, using familiar mobile devices to upload health data, and telemedicine calls with a doctor are the elements of decentralized clinical trials with the biggest positive impact on participation in future studies, according to a survey.

Advarra
MARCH 6, 2025
Food and Drug Administrations (FDA) regulations on good clinical practice, which are based on ethical standards outlined in frameworks such as the Belmont Report and the Declaration of Helsinki. Advarra ensures that clinical trials comply with the U.S.

The Pharma Data
JANUARY 21, 2021
Eli Lilly reported yesterday that its monoclonal antibody bamlanivimab (LY-CoV555) significantly lessened the risk of contracting COVID-19 among nursing home residents and staff, according to findings from a phase 3 trial. James Miessler. Source link.

Trialfacts
JULY 5, 2024
Researchers from The University of Arizona College of Nursing want to understand how to better support survivors like you. About the Research Center The University of Arizona College of Nursing The University of Arizona—one of the country’s most respected research institutions—is a leader in health-sciences education and scholarship.

FDA Law Blog
JUNE 22, 2022
In the second case, occurring from January to May 2020, a registered nurse, admitted to daily tampering of fentanyl vials and hydromorphone injectables wherein she removed the drugs from the vials replacing them with saline solution. It is worth noting that the nurse was separately sentenced to 4.5 4, 2022). 4, 2022).

XTalks
DECEMBER 22, 2022
Home visits by nurses or other research staff. Neilan: Application of the rapidly evolving new technologies or approaches is limited somewhat by lack of awareness or familiarity on the part of investigators and/or regulators, either of which may result in defaulting to older, “proven” means. video call) study visits.

Pharma Marketing Network
SEPTEMBER 14, 2020
Give your staff, reps, and other KOLs the chance to create personalized videos to thank local HCPs, nurses, and others on the front line of the coronavirus crisis. For the heavily-regulated healthcare industry, these platforms help maintain compliance with built-in processes and tools to ensure proper permissions are granted at each stage.

ACRP blog
OCTOBER 8, 2024
Food and Drug Administration (FDA) has released two Notices of Proposed Rulemaking (NPRMs), the initial public notice of a proposed change to federal regulations. The Common Rule was revised in 2018, and the FDA’s two recent NPRMs will harmonize the FDA regulations with most of those 2018 revisions. What is the Single IRB Mandate?

ACRP blog
FEBRUARY 26, 2024
The irony―I am a cancer nurse and have been all my career. …My Just this year, Penn State Cancer Institute has participated in 10 treatment trials for breast cancer alone.” Saying that, taking care of cancer patients is all she ever wanted to do since graduating from nursing school in 1986.

ACRP blog
FEBRUARY 29, 2024
Duke University’s Schools of Medicine and Nursing currently employ more than 900 clinical research professionals, with around 200 new hires per year. ” UAB breaks its Clinical Research Career Ladder into five tracks or types of roles—administration, coordinator, data, nurse coordinator, and regulatory, explains Marchant.

pharmaphorum
JUNE 15, 2022
Astellas has a window of opportunity to build awareness and use of Evrenzo in Europe ahead of GlaxoSmithKline’s daprodustat – which has just been filed for approval there – and Otsuka/Akebia Therapeutics’ vadadustat which was submitted to regulators last October.

pharmaphorum
NOVEMBER 1, 2021
Like most heavily regulated industries, pharma is very process driven, but patient centricity requires the focus to be on outcomes, rather than tasks. “We As well as digital tools and nurse visits, they sometimes wanted to see the doctor,” she said. How are you making patient centricity a reality? We were very proud of it.

XTalks
FEBRUARY 22, 2021
Clinical research coordinators must ensure regulations are met and both safety and governmental rules are applied. Once that is completed and the trial begins, the clinical research coordinator will be responsible for ensuring that all team members are compliant with the rules and regulations.

XTalks
AUGUST 9, 2024
Technological advances, changing patient preferences and evolving guidance from regulators and policy makers have all supported new research methods that break down barriers to participation. Pharmaceutical, life sciences and healthcare organizations have long had the desire to make their research programs more accessible and patient-centric.

XTalks
AUGUST 12, 2020
This advice applies to all individuals across Canada, as well as retailers, distributors, manufacturers and food service establishments such as hotels, restaurants, cafeterias, hospitals and nursing homes.”.

XTalks
AUGUST 25, 2021
While the bars were not given out to patients, they were given to most hospital workers, from security to nurses, to doctors and administrative staff. “Them paying for the bars at cost allowed us to continuously manufacture, which allowed us to keep going and keep our team in stock.”.

ACRP blog
SEPTEMBER 12, 2024
Celaya: Non-traditional students, many of whom come from established careers in healthcare or related fields, bring practical experience to the table, often in areas like nursing, clinical areas, data management, or regulatory affairs. Many of them come to us from entry-level clinical research jobs.

Pharmacy Checkers
NOVEMBER 21, 2019
As my greater advocacy initiatives have worked to implore the FDA to bring more balance, commonsense and fairness to regulating and providing consumer education about personal imports of prescription drugs, I’ve realized that I should practice the same in how I talk about the FDA. The medications that we obtain in the U.S.,

The Pharma Data
OCTOBER 24, 2020
“It’s a very traumatic event,” said Debbie Medley, an assistant nurse manager in the emergency department at Penn State Health Medical Center. Even when attackers aren’t known, more than half the victims do not report their assaults.
The Pharma Data
AUGUST 28, 2020
The Department of Health and Social Care (DHSC) said this could include training a wider range of existing NHS staff, as well as student doctors and nurses. Proposals, which are being considered in a three-week consultation , are designed to cut red tape and prevent any delays between a vaccine being found and then given to the UK population.

ACRP blog
NOVEMBER 13, 2024
Visit the ACRP Guidelines and Regulations Resources Center for help and perspectives along the way. Begin by reviewing your current training programs to ensure they are aligned with the changes expected under ICH E6(R3). How will you train new employees and incorporate continuous training? She is also the ACRP Ohio Chapter President.

XTalks
JANUARY 8, 2024
This form connects patients with the full array of benefits provided by Tarpeyo Touchpoints, including financial aid programs designed to reduce or completely cover out-of-pocket costs, access to Calliditas’ team of care navigators, pharmacists and nurse educators, and the advantage of convenient at-home, next-day delivery.

Pharma Marketing Network
SEPTEMBER 14, 2020
Give your staff, reps, and other KOLs the chance to create personalized videos to thank local HCPs, nurses, and others on the front line of the coronavirus crisis. For the heavily-regulated healthcare industry, these platforms help maintain compliance with built-in processes and tools to ensure proper permissions are granted at each stage.

Advarra
SEPTEMBER 7, 2023
Together, these three principles form the foundation upon which HRPPs were established and provide the basis for laws, regulations, guidelines, and policies governing human subjects research. They must ensure research is conducted in compliance with the protocol and applicable policies and regulations.
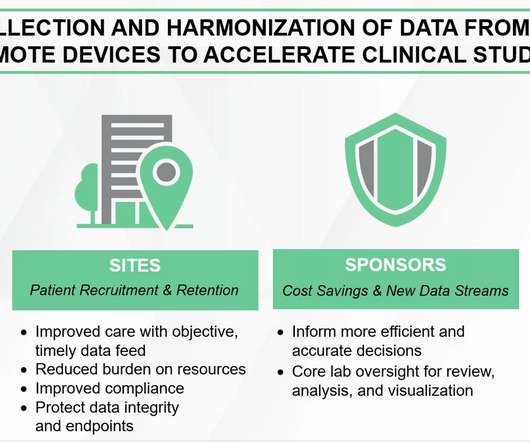
XTalks
JANUARY 19, 2022
For instance, most of the surveyed investigators expect telemedicine consultation, remote patient monitoring, remote site-initiation visits, electronic consent and in-home nurse visits will continue to be important DCT services in clinical trials after the pandemic. Incorporating Wearable Biosensors in DCTs.

Pharmacy Checkers
JANUARY 10, 2020
We know there are many doctors, nurses and other prescribing medical professionals who recommend PharmacyChecker or have learned of trusted licensed foreign pharmacies though their own research. France, according to the FDA, is recognized as having similarly strict pharmaceutical regulations to the U.S., Is the drug safe?

Crucial Data Soutions
NOVEMBER 16, 2022
With recruitment and retention of study participants remaining the top challenge for most sponsors, and with regulators giving increasingly pointed guidance around the need to improve diversity and representation in research, many study designers are looking at DCT approaches to help bring trials closer to patients.

Trialfacts
OCTOBER 15, 2023
More Study Details About the Research Center: Research Center: University of Texas at Austin (School of Nursing) Location: This is a nationwide virtual study. Participants will be randomized to one of two groups to receive stress, emotional regulation, and support intervention using a mobile health app. Your Rights Who Can Participate?

Trialfacts
APRIL 12, 2023
More Study Details About the Research Center: Research Center: University of Texas at Austin (School of Nursing) Location: This is a nationwide virtual study. Participants will be randomized to one of two groups to receive stress, emotional regulation, and support intervention using a mobile health app. Your Rights Who Can Participate?

XTalks
MAY 12, 2023
The MeCP2 protein plays a crucial role in regulating the activity of genes involved in brain development. As we launched the drug, we, along with some of the clinicians and nurses in the field, instituted a diarrhea management plan to help the caregivers deal with the diarrhea.

Cloudbyz
MARCH 12, 2023
Review documentation Review the site’s regulatory and institutional review board (IRB) documentation to ensure that it is up to date and in compliance with local regulations. The meeting is an opportunity to establish rapport with the site staff and to build a positive working relationship.

The Pharma Data
DECEMBER 4, 2020
women who were part of the long-running Nurses’ Health Study II. But in theory, there’s an explanation, she said: Sunlight — and specifically the ultraviolet B radiation it emits — spurs the body to produce vitamin D, which is anti-inflammatory and helps regulate immune function.

Pharma Marketing Network
DECEMBER 19, 2024
Healthcare Providers: Key Influencers Physicians, nurses, and pharmacists are the gatekeepers of medical advice and treatment recommendations. Regulatory Compliance In an industry as heavily regulated as pharma, compliance is non-negotiable. Overcoming these obstacles is key to achieving impactful results.

Delveinsight
FEBRUARY 17, 2021
Besides it is in use to analyze population health trends, medical records, virtual nursing assistants, and administrative work. The government also needs to bring strict and standard legislation and regulations to deal with data breaches in healthcare and other sectors. . Healthcare Mobile Apps (Medical mobile applications).

XTalks
FEBRUARY 3, 2021
The recommendations were made by experts in pediatrics, infectious diseases, immunology, pharmacy, nursing, epidemiology, pharmacoeconomics, social science and public health. I did not want anything in the future, such as regulations, to stop me from being able to reach my daughters who reside in another country if I needed to.

Advarra
APRIL 30, 2024
In some countries, local regulations may indicate the sub-investigators need to sign as well. Both the medical licenses and nurse licenses need to be up to date. Screening/enrollment log: Confirm all screened participants are present on the log and the reason for screen failure or date of randomization recorded.
XTalks
FEBRUARY 10, 2023
It is important to have a comprehensive Product Development Plan (PDP) that is developed early and in collaboration with regulators and the non-clinical, manufacturing, clinical operations and logistics teams. Medpace Regulatory Team support throughout product development. If so, why?

ACRP blog
MAY 17, 2023
Sidebar 2: Informal Poll at ACRP 2023 Finds Regulations to be “Clear as Mud!” Nearly 50% said the regulations were “clear as mud” and nearly 50% wished “someone would send help” for dealing with the challenges faced. The draft guidance offers welcome clarity that this is still the case for DCTs.

Roots Analysis
FEBRUARY 21, 2024
In addition, several exoskeletons can help healthcare workers, such as doctors and nurses in physically demanding tasks and repetitive work to avoid back injuries. The exoskeleton market is witnessing substantial growth, propelled by technological advancements in both medical and non-medical domains.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content