FDA approves Amneal and Shilpa’s oncology product BORUZU
Pharmaceutical Technology
SEPTEMBER 6, 2024
The US FDA has approved Amneal Pharmaceuticals and Shilpa Medicare’s oncology product BORUZU for subcutaneous administration.
 Production Related Topics
Production Related Topics 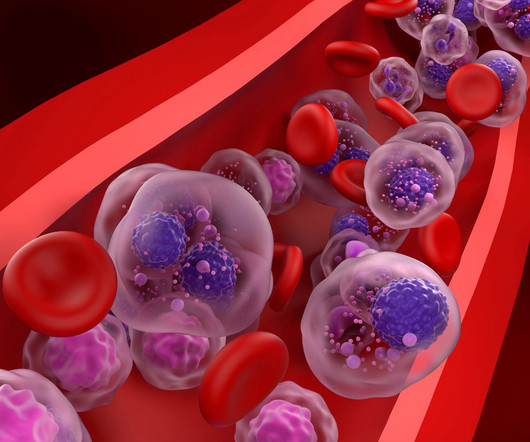
Pharmaceutical Technology
SEPTEMBER 6, 2024
The US FDA has approved Amneal Pharmaceuticals and Shilpa Medicare’s oncology product BORUZU for subcutaneous administration.
Pharmaceutical Technology
APRIL 13, 2023
The now expanded agreement will enable Rubic to explore other therapeutic avenues in both the human and animal health product markets. The extension of the agreement beyond its original Covid-19 focus will accelerate development timelines, increase productivity, and lower manufacturing costs, according to Dyadic’s CEO Mark Emalfarb.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

AuroBlog - Aurous Healthcare Clinical Trials blog
APRIL 3, 2024
Evidence does not support the use of cannabidiol (CBD) products as a treatment for chronic pain, a new review found.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 29, 2024
Click here Diagnostics + Font Resize – NABL launches comprehensive training programme to enhance competence in reference material production Shardul Nautiyal, […]

Advertisement
Sponsors can face a number of challenges when trying to obtain these critical supplies for their studies, including addressing product lead times, availability, expiry limitations, safety and cost, among others.

AuroBlog - Aurous Healthcare Clinical Trials blog
APRIL 9, 2024
Exports of Ayush (Ayurveda, Yoga, Naturopathy, Unani, Siddha, Sowa-Rigpa and Homoeopathy) drugs and herbal products from the country have reported 1.65 per cent growth during the first ten months of the current fiscal year. The growth in the month of January stood at 14.54

XTalks
OCTOBER 23, 2024
Oxford Medical Products has shared promising safety data from a first-in-human study for its ‘mechanical’ weight loss pill. Oxford’s device showed a favorable safety profile, with no serious adverse events reported, and no participants withdrew from the study due to product-related issues.
Advertisement
As demand for advanced therapies increases, so does the need for more specialized supply chain support, as these products have strict transportation and handling requirements.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight.
Let's personalize your content