Pfizer set to double weekly production of coronavirus vaccine
Bio Pharma Dive
FEBRUARY 19, 2021
The drugmaker will use production lines at its plant in McPherson, Kansas to help fill vaccine vials, company CEO Albert Bourla said Friday.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
FEBRUARY 19, 2021
The drugmaker will use production lines at its plant in McPherson, Kansas to help fill vaccine vials, company CEO Albert Bourla said Friday.
Pharmaceutical Technology
APRIL 13, 2023
The expanded licence will include the development of vaccines and therapeutic proteins beyond Covid-19 for human and animal health markets in Africa. During the Covid-19 pandemic, vaccination rates of many countries in Africa were significantly trailing the rest of the world.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
MAY 23, 2023
Croda Pharma has entered a strategic collaboration deal with Botanical Solution Inc (BSI) to expedite the production of sustainable pharmaceutical-grade vaccine adjuvant QS-21. The plentiful supply of QS-21 enables the production of next-generation adjuvant systems for new vaccine development.”
Bio Pharma Dive
MAY 4, 2021
The sum is $11 billion higher than Pfizer had estimated in February and, if realized, would make the vaccine the highest grossing pharmaceutical product by revenue in a single year.

Bio Pharma Dive
DECEMBER 4, 2020
Moncef Slaoui, a leader of Operation Warp Speed, said he expects 100 million Americans to be vaccinated by the end of February — an ambitious target even as Moderna and Pfizer ramp up manufacturing.

Pharmaceutical Technology
SEPTEMBER 27, 2024
Bavarian Nordic has already said it would prioritise mpox vaccine production to fulfil orders this year.

World of DTC Marketing
DECEMBER 29, 2020
OBSERVATION: Biologics can take a long time to develop but COVID vaccines have been in development for almost 50 years and novel approaches were used to develop these vaccines. Vaccines typically take 10 to 15 years to develop, test and release to the public. The coronavirus vaccines, however, took less than a year.

Bio Pharma Dive
SEPTEMBER 24, 2020
HHS Secretary Alex Azar said six vaccine developers involved with Operation Warp Speed had begun commercial manufacturing of their experimental shots.

Bio Pharma Dive
APRIL 5, 2021
The change, which follows the contractor's costly manufacturing mix-up, will displace production of AstraZeneca's unauthorized shot at the Baltimore site.
Pharmaceutical Technology
SEPTEMBER 7, 2022
Indian company Bharat Biotech International has secured approval from Central Drugs Standard Control Organisation (CDSCO) under Restricted Use in Emergency Situation for its intranasal Covid-19 vaccine, iNCOVACC (BBV154), for usage in people aged 18 years and older. It is formulated to permit intranasal delivery via nasal drops.
Pharmaceutical Technology
MAY 30, 2023
SK Bioscience has received marketing authorisation from the UK’s medicines and healthcare products regulatory agency (MHRA) for its Covid-19 vaccine, SKYCovion. The authorisation allows the distribution of the vaccine in Scotland, Wales and England.

Bio Pharma Dive
JULY 25, 2022
Vaccine maker Bavarian Nordic said it is meeting demand for its shot with production capacity of 30 million doses a year.

Pharmaceutical Technology
MARCH 28, 2023
Following on from its Covid-19 vaccine programmes, BioNTech has set its sights on a range of infectious diseases for vaccine development. The company saw major successes with its Covid-19 vaccine, developed in collaboration with Pfizer. In response to the lower vaccine sales forecasts, BioNTech shares opened at 3.9%

Bio Pharma Dive
AUGUST 26, 2022
The biotech claims its rivals’ vaccine Comirnaty, one of the world’s top-selling pharmaceutical products, infringes on two patents covering its messenger RNA technology.
Pharmaceutical Technology
FEBRUARY 7, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorisation for Takeda ’s dengue virus vaccine candidate, Qdenga (Dengue Tetravalent Vaccine [Live, Attenuated]). The vaccine candidate has been approved for active immunisation against the infection in people from four years of age.
Pharmaceutical Technology
SEPTEMBER 5, 2022
The National Medical Products Administration of China (NMPA) has approved CanSino Biologics ’ (CanSinoBIO) recombinant Covid-19 vaccine (Adenovirus Type 5 Vector) for inhalation, Convidecia Air, as a booster. This vaccine leverages the same adenovirus vector technological platform as Convidecia, the intramuscular version.
Pharmaceutical Technology
AUGUST 1, 2022
Moderna has entered a new supply contract with the US Government to deliver 66 million doses of its Covid-19 vaccine booster candidate, mRNA-1273.222. The contract comprises a $1.74bn award to produce and supply these vaccine doses and options to further procure up to 234 million additional doses of the company’s booster candidates.

Pharmaceutical Technology
MARCH 11, 2025
MSD has opened a $1bn facility in Durham located in the US state of North Carolina, to increase vaccine manufacturing capacity.

Pharmaceutical Technology
AUGUST 2, 2022
Samsung Biologics and GreenLight Biosciences have completed the initial commercial-scale engineering run for their messenger ribonucleic acid (mRNA) Covid-19 vaccine under their manufacturing collaboration. Following the demonstration at Samsung, the clinical trial of GreenLight’s Covid-19 booster vaccine is anticipated to commence this year.

World of DTC Marketing
JANUARY 8, 2021
SUMMARY: Pfizer and Moderna will sell $28 billion of Covid-19 vaccines this year. Pharma companies will make an estimated $40 billion on the global COVID-19 vaccine market this year, which will be split between Johnson & Johnson, AstraZeneca, Novavax, and others. Can the FDA really control the quality of vaccine orders this big?
Pharmaceutical Technology
DECEMBER 11, 2022
The US Food and Drug Administration (FDA) has granted Fast Track designation for Pfizer and BioNTech’s messenger ribonucleic acid (mRNA)-based combination vaccine candidate against Covid-19 and influenza. The vaccine is intended to prevent two respiratory ailments through a single injection. 5 Omicron sublineages spike proteins.

Pharmaceutical Technology
JANUARY 13, 2023
The Biomedical Advanced Research and Development Authority (BARDA) has awarded a multi-year contract to the Sabin Vaccine Institute for developing and producing single-dose vaccine candidates for Ebola Sudan and Marburg virus diseases. Currently, there are no licensed vaccines against these viruses.

Bio Pharma Dive
FEBRUARY 24, 2021
The biotech has delivered a newly developed booster shot to NIH scientists, and will test several other approaches to address emerging virus strains.
Pharmaceutical Technology
FEBRUARY 3, 2023
Only a few weeks into the new year, the prospect of getting a successful advanced HIV vaccine shrank after the discontinuation of yet another late-stage trial. On January 18, Janssen, a Johnson & Johnson (J&J) subsidiary, stated that its vaccine was not effective in preventing HIV infections.

Pharmaceutical Technology
JULY 6, 2022
The European Commission (EC) has granted approval for the expanded conditional marketing authorization (CMA) of Novavax’s Covid-19 vaccine, Nuvaxovid (NVX-CoV2373), in the European Union (EU) for adolescents of the age 12 to 17 years. A protein-based vaccine, NVX-CoV2373 is made from the genetic sequence of the SARS-CoV-2 virus’ first strain.

Pharmaceutical Technology
AUGUST 1, 2022
GreenLight Biosciences has entered a partnership with the US National Institutes of Health (NIH) for the development of Covid-19 vaccines, which offer broader protection against new variants and with durable effects. They intend to develop vaccines that provide lasting immune responses compared to existing vaccines.

Pharmaceutical Technology
JUNE 24, 2022
Novavax has obtained emergency use authorization (EUA) for its Covid-19 vaccine, Nuvaxovid (NVX-CoV2373), from the Taiwan Food and Drug Administration for use in people of the age 18 years and above. The protein-based vaccine is engineered from the genetic sequence of the SARS-CoV-2 virus’ initial strain.

Bio Pharma Dive
JULY 27, 2023
GSK expects its respiratory syncytial virus vaccine will be a multibillion dollar product. But at the beginning it’s predicting a slower launch than for its fast-selling shingles shot.
Pharmaceutical Technology
JULY 15, 2022
Health Canada has granted approval for the usage of Moderna’s messenger RNA (mRNA) Covid-19 vaccine, Spikevax, in a 25µg two-dose regimen for active immunisation to prevent Covid-19 in children aged six months to five years. So far, children aged below five years were not eligible to receive the Covid-19 vaccine in Canada.

Bio Pharma Dive
SEPTEMBER 3, 2020
The planned $50 million infusion is the third investment in Catalent's Bloomington facility in two years, reflecting the need for vial filling for biologic drugs and vaccines.

Pharmaceutical Technology
MARCH 20, 2023
RVAC Medicines has announced a research collaboration with the University of Pennsylvania (Penn) for the discovery and development of mRNA vaccines. The mRNA vaccine candidates will help reduce the chances of autoimmune responses that might lead to allergic conditions or serious autoimmune diseases.

Bio Pharma Dive
MAY 31, 2022
The deal is the second acquisition for GSK since April and gives the company access to an experimental shot that’s meant to surpass new products from Pfizer and Merck & Co.
Pharmaceutical Technology
FEBRUARY 23, 2023
GenScript ProBio has announced a strategic collaboration with RVAC Medicines to manufacture GMP-grade plasmid DNA (pDNA) for the latter’s RVM-V001, an mRNA Covid-19 vaccine candidate. The post GenScript ProBio and RVAC partner for Covid-19 vaccine pDNA appeared first on Pharmaceutical Technology.
Pharmaceutical Technology
JUNE 20, 2023
The World Health Organisation (WHO) has granted an emergency use listing (EUL) to SK bioscience’s Covid-19 vaccine, SKYCovione. SKYCovione is a self-assembled nanoparticle vaccine and the 12th Covid-19 vaccine to receive a EUL from the regulator.

Bio Pharma Dive
NOVEMBER 3, 2022
The biotech dialed back its financial projections after reporting lower-than-expected sales for the shot, which currently remains the company’s only marketed product.

Fierce Pharma
OCTOBER 9, 2023
Following similar initiatives to bolster mRNA vaccine production and access in Africa, the Bill & Melinda Gates Foundation is providing new funding to a clutch of drugmakers.
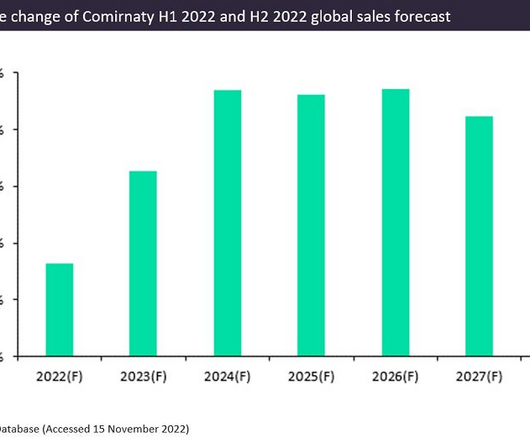
Pharmaceutical Technology
FEBRUARY 21, 2023
Pfizer and BioNTech’s Covid-19 vaccine, Comirnaty, had a phenomenal year with forecast sales of $37bn in 2022. Comirnaty is the leading prophylactic vaccine for Covid-19 and is expected to generate an additional $2.8bn in sales in 2022 compared to GlobalData’s H1 2022 forecast. Comirnaty is the first globally approved Covid-19 vaccine.

Bio Pharma Dive
FEBRUARY 11, 2021
The drugmaker says between six to nine months are needed to ramp up production of an adapted shot. South Africa recently stopped using the vaccine due to its reduced potency against a common strain there.
Pharmaceutical Technology
AUGUST 15, 2022
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted conditional authorisation for Moderna ’s Covid-19 booster vaccine, mRNA-1273.214 (Spikevax Bivalent Original/Omicron), for use in adults aged 18 years and above. In addition, no serious safety concerns linked to the vaccine were observed.

Bio Pharma Dive
JULY 5, 2023
The vaccine has a chance to become Moderna’s second approved product and could help offset declining sales for its COVID-19 shot.

Pharmaceutical Technology
MAY 25, 2023
A new vaccine developed by the Serum Institute of India to fight meningococcal disease could help eliminate meningitis across Africa. The results from a trial, published in The New England Journal of Medicine , found the vaccine was associated with a strong immune response and good safety profile. percentage points (96% CI, −0.3

Bio Pharma Dive
OCTOBER 31, 2023
The pharma’s shot Abrysvo made over $300 million in sales during the first few months of its launch, and will be an important product to help offset declining COVID vaccine sales.

Bio Pharma Dive
SEPTEMBER 13, 2024
During an investor presentation Thursday, executives admitted to being overly optimistic their vaccine could wrest significant market share away from GSK's and Pfizer's products this year.

Pharma Mirror
MAY 23, 2023
QS-21 is used in several innovative vaccines against diseases such as shingles, malaria and RSV, plus promising new vaccine and immunotherapy treatments such as cancer. BSI) partner to fast-track sustainable vaccine adjuvant QS-21 production appeared first on Pharma Mirror Magazine.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content