Wave sees RNA editing validation in early trial results
Bio Pharma Dive
OCTOBER 16, 2024
The results provide the first clinical evidence of RNA editing, a burgeoning field that's drawn interest from biotechs and pharmaceutical companies alike.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
OCTOBER 16, 2024
The results provide the first clinical evidence of RNA editing, a burgeoning field that's drawn interest from biotechs and pharmaceutical companies alike.
Pharmaceutical Technology
MAY 11, 2023
The vaccine, developed by BioNTech, led to half of the patients with pancreatic cancer in the Phase I trial remaining cancer-free 18 months later. A global randomised follow-up trial is planned. In 2016, BioNTech and Genentech, a part of Roche signed an agreement to develop personalised mRNA therapies in oncology.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
FEBRUARY 10, 2023
The pharma and partner BioNTech have kicked off the first human trial of a messenger RNA shot for shingles, believing there’s room to clear the high bar set by GSK’s rival vaccine.

Pharmaceutical Technology
JULY 29, 2022
Last week, CAMP4 Therapeutics announced the close of a $100 million Series B round , which will be used to advance their regulatory RNA (regRNA)-focused programs. CAMP4’s CSO David Bumcrot PhD tells Pharmaceutical Technology that the company plans to see clinical trials go forward for their urea cycle disorder programs late next year.

Bio Pharma Dive
JUNE 17, 2024
Elevate precision medicine: dose optimization and immune monitoring through advanced liquid biopsies.

Pharmaceutical Technology
JUNE 16, 2023
from Flanders Innovation & Entrepreneurship (VLAIO) to further advance its oncology portfolio targeting RNA. It will also support the firm’s preclinical effort on the long non-coding RNA (LncRNA) programme, FTX-001, that targets MALAT-1. Flamingo Therapeutics has received a research grant of €1.7m

Bio Pharma Dive
MAY 19, 2023
The biotech, best known for its vaccine research, said its clinical trial is the first to report results of a messenger RNA therapeutic designed for intracellular protein replacement.
Pharmaceutical Technology
MARCH 1, 2023
Further, the acceptance of new mRNA vaccines has rejuvenated activity within previously established categories of RNA therapeutics including lifesaving antisense technologies. These include antisense oligonucleotides (ASO), RNA interference (RNAi), and RNA aptamers.

Bio Pharma Dive
APRIL 1, 2025
Airna will use the Series B round to launch a Phase 1/2 trial of its lead program for alpha-1 antitrypsin deficiency, a disease targeted by several other firms.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 1, 2023
The Drug Controller General of India (DCGI) has added in-vitro diagnostic (IVD) medical devices including those for diagnosis of Covid-19, ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) extraction kits, among others into the Class C risk category under the Medical Devices Rules (MDR), 2017.

pharmaphorum
MARCH 25, 2024
billion, adding expertise in RNA therapeutics and a heart failure therapy in mid-stage trials. Novo Nordisk has agreed to buy Cardior Pharmaceuticals for $1.1

pharmaphorum
MAY 2, 2024
A personalised vaccine for the aggressive brain cancer glioblastoma developed has shown encouraging signs of efficacy in its first human trial.

Pharmaceutical Technology
MARCH 28, 2023
There is much interest in the industry around RNA-based therapeutics as their utilisation in indications beyond Covid-19 come into focus. In December 2022, BioNTech initiated a Phase I clinical trial of BNT163 – an HSV vaccine candidate. In 2022, the company also started five first-in-human clinical trials from its oncology programme.

XTalks
SEPTEMBER 13, 2021
With RNA therapies being the next hot thing in genetic medicine, Eli Lilly is joining the RNA editing race by partnering with Netherlands-based ProQR Therapeutics NV (Nasdaq: PRQR), a biotech company developing RNA-based therapies for rare genetic diseases with a focus on blinding disorders of the retina.

Pharmaceutical Technology
MAY 15, 2024
CircRNA is still in early days of development, but could be in trials as vaccines, therapeutics and biomarkers in the next few years.

Pharma Mirror
SEPTEMBER 2, 2021
In-vitro studies conducted at the British virology research laboratory Virology Research Services in London then demonstrated a potent antiviral activity against SARS-CoV-2 and other RNA viruses. The trial was conducted at. The post Codivir Shows Promising Effect Against COVID-19 appeared first on Pharma Mirror Magazine.

pharmaphorum
AUGUST 16, 2022
Merck & Co has ramped up its involvement in the RNA category, partnering with US biotech Orna Therapeutics in a deal valued at up to $3.5 Now, Merck has made its own play, partnering with Orna on its proprietary ‘oRNA’ technology, which stands for circular RNA. billion, including $150 million upfront.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 16, 2022
The husband-and-wife team who co-founded BioNTech, the biotechnology company that partnered with Pfizer to develop an effective messenger-RNA (mRNA) shot against COVID-19, has predicted that a cancer vaccine could be widely available within the next decade.

XTalks
MARCH 21, 2022
Credited as a pioneering RNA interference (RNAi) therapeutics company, Alnylam Pharmaceuticals is taking Pfizer and Moderna to court, claiming that the companies’ use of the lipid nanoparticle (LNP)-based RNA delivery technology in their mRNA COVID-19 vaccines infringes on a patented technology.

Bio Pharma Dive
AUGUST 11, 2022
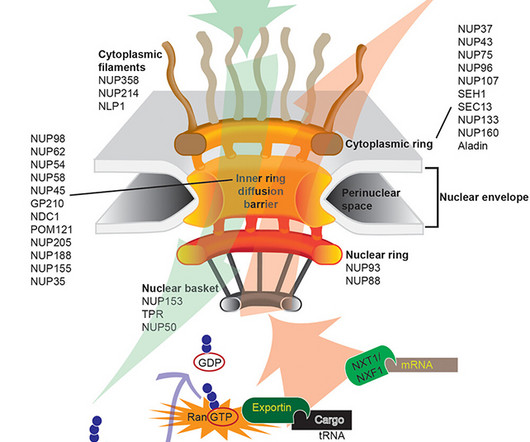
The company will wind down five trials involving two drug candidates and lay off more staff, prioritizing instead its RNA-editing technology.
AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 11, 2025
Researchers from Arizona State University suggest that stress granules protein and RNA clumps that form around cells in stressful […]

AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 17, 2024
We are witnessing a revolution in healthcare, driven by advances in genetics, Omics, RNA and CRISPR gene-editing technology, to deliver precision and personalised medicine, said Kiran Mazumdar-Shaw, executive chairperson, Biocon and Biocon Biologics. Biology is opening up new frontiers in medicine.
Pharmaceutical Technology
OCTOBER 14, 2022
In 2016, the companies entered a strategic partnership to develop novel messenger RNA (mRNA) based PCVs. It is currently being assessed in combination with Merck’s anti-PD-1 therapy, Keytruda, as an adjuvant treatment for high-risk melanoma patients in a Phase II clinical trial being conducted by Moderna.

Fierce Pharma
JUNE 24, 2024
In one of the most closely watched biotech trial readouts of the year, Alnylam Pharmaceuticals said its RNA interference drug reduced the risk of death or recurrent cardiovascular events in patient | In one of the most closely watched biotech trial readouts of the year, Alnylam Pharmaceuticals said its RNA interference drug reduced the risk of death (..)

Pharmaceutical Technology
AUGUST 15, 2024
Clinical trials for the two RNA interference (RNAi) programmes are slated to commence in early 2025.
Pharmaceutical Technology
SEPTEMBER 16, 2022
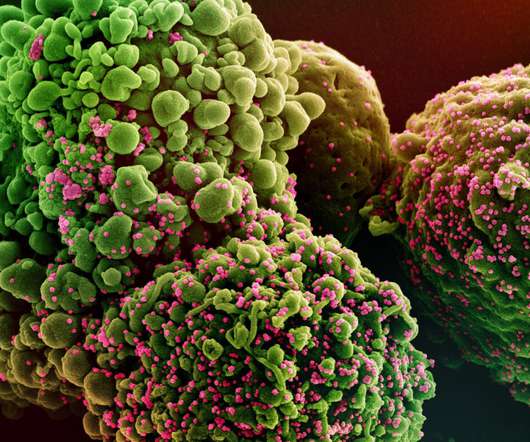
The latest conditional recommendation is based on the final data from the SOLIDARITY clinical trial, which was sponsored by the WHO. These results are in line with those obtained from the placebo-controlled, double-blind ACTT-1 trial of the National Institute of Allergy and Infectious Diseases.
STAT News
JANUARY 9, 2023
An experimental RNA treatment reduced liver scarring in half of patients with an inherited disease called alpha-1 antitrypsin deficiency, or AATD, according to results from a mid-stage clinical trial reported Monday by its maker Arrowhead Pharmaceuticals.
Pharmaceutical Technology
JULY 15, 2022
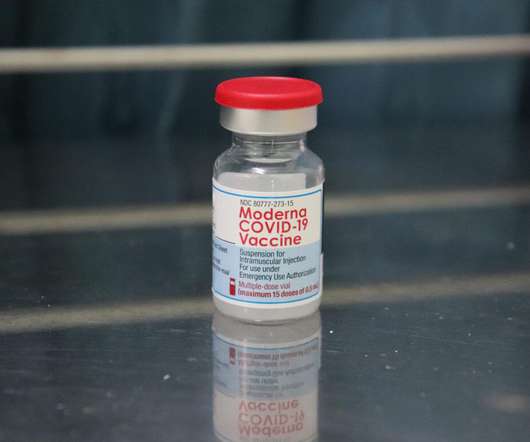
Health Canada has granted approval for the usage of Moderna’s messenger RNA (mRNA) Covid-19 vaccine, Spikevax, in a 25µg two-dose regimen for active immunisation to prevent Covid-19 in children aged six months to five years. The KidCOVE trial was carried out at eight Canadian trial sites involving 414 children aged below five years.

XTalks
DECEMBER 4, 2024
Proceeds from the IPO will propel key initiatives, including the Phase II clinical trial of Jotrol in Parkinson’s disease. While Jupiter faces financial uncertainties and challenges in scaling operations in competitive markets, it remains committed to refining grant applications for proof-of-concept trials.

Pharmaceutical Technology
NOVEMBER 8, 2022
Additionally, Neurophth will oversee the clinical trials and marketing of gene therapy products developed leveraging the new AAV capsids of Cyagen. Both companies will assess the new AAV vectors’ functional effects in rodent and NHP models.

pharmaphorum
MARCH 3, 2024
Avidity Biosciences files $400m private placement as it prepares for phase 3 trial of lead antibody oligonucleotide conjugate AOC 1001 in rare disease DM1
Pharmaceutical Technology
JANUARY 19, 2023
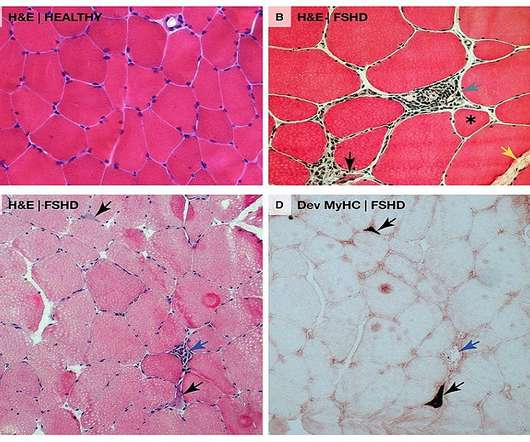
“We look forward to working collaboratively with the FDA to bring the first RNA therapy directly targeting DUX4 to patients as quickly as possible.” AOC 1020 is currently being studied in the double-blind, randomised, placebo-controlled Phase I/II FORTITUDE clinical trial in nearly 70 FSHD adult patients.

World of DTC Marketing
DECEMBER 29, 2020
Both the Pfizer and Moderna vaccines copied RNA sequence from the virus genome and found a way to manufacture it at scale with high-level processes and quality control. Another consideration is that while in traditional vaccine development, clinical trials are carried out in sequence. 1455NO-HEALTH-CORONAVIRUS_VACCINES_PFIZER_O_.
Pharmaceutical Technology
MAY 19, 2023
Myeloid Therapeutics has raised $73m to support the continued clinical development of its lead cell therapy programme, MT-101, in Phase I/II trials for T cell lymphoma. The financing will help to fast-track the development of other in vivo programming candidates into clinical trials.

Pharmaceutical Technology
AUGUST 2, 2022
The RNA platform of Samsung Biologics permitted GreenLight to move from mRNA vaccine conceptualisation to the delivery of released clinical trial material in under two years. Following the demonstration at Samsung, the clinical trial of GreenLight’s Covid-19 booster vaccine is anticipated to commence this year.

Pharmaceutical Technology
APRIL 3, 2023
GlobalData, the parent company of Pharmaceutical Technology, outlines a rise in gene therapy trials in 2023, in line with the growing market Fáber describes. According to GlobalData’s Clinical Trials Database, gene therapy trials represent a 0.91% share of all planned trials in 2023, up from just 0.25% in 2014.
Pharmaceutical Technology
FEBRUARY 3, 2023
Only a few weeks into the new year, the prospect of getting a successful advanced HIV vaccine shrank after the discontinuation of yet another late-stage trial. This marks the second time one of Janssen’s HIV vaccines failed after another showed disappointing results in the Phase IIb Imbokodo trial in August 2021.

XTalks
JANUARY 8, 2024
Whether it’s for a treatment for a chronic ambulatory condition, precision medicine or cell and gene therapy, there is a massive uptick in clinical trial complexity. It’s important to make sure that with this increase in clinical trial complexity, we don’t make our trials overly burdensome to sites or patients,” says Markham.

pharmaphorum
SEPTEMBER 2, 2020
Spain’s Highlight Therapeutics is attempting to get patients with melanoma to respond to Keytruda by combining with its RNA-based cancer therapy in patients with melanoma who have progressed despite treatment with PD-1 therapies such as Keytruda or BMS’ rival Opdivo (nivolumab). A total of 40 patients are planned to be enrolled.

STAT News
JANUARY 30, 2023
It is now possible to treat diseases with gene therapy, antisense oligonucleotides, messenger RNA (mRNA), noncoding RNA (known as small interfering RNA, or siRNA), and other gene-based modalities. New ways of conducting clinical trials have also emerged. The human genome was sequenced in 2003. Read the rest…

Fierce Pharma
FEBRUARY 15, 2024
Changing a clinical trial’s statistical analysis plan on the cusp of a readout? Changing a clinical trial’s statistical analysis plan on the cusp of a readout? That’s exactly what Alnylam just did for a closely watched study of its next-generation RNA interference therapy Amvuttra in a rare heart disease.
Pharmaceutical Technology
JUNE 30, 2022
The vaccine leverages self-amplifying RNA (saRNA), which can replicate itself after administration and could be effective at very low doses. At present, the vaccine is being analysed in clinical trials in the US, Brazil and South Korea. HDT Bio CEO Steve Reed said: “Our saRNA vaccine is a game-changer.
Pharmaceutical Technology
SEPTEMBER 1, 2022
These 'updated boosters' comprise the SARS-CoV-2 virus’ two messenger RNA (mRNA) components, one from the initial strain and the other one common between the BA.4 According to the amended EUA, the vaccines are indicated to be administered at a minimum of two months after the initial or booster dose. 5 lineage of the Omicron variant.
pharmaphorum
OCTOBER 27, 2020
US biotech BioSig Technologies has abandoned a phase 2 trial of its antiviral drug merimepodib with Gilead’s Veklury in severe COVID-19 patients, after concluding the safety of the combination was in doubt. However the Grade 3 group had “markedly different outcomes… making it unlikely that the trial would meet its primary safety endpoints.”.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content