GSK hires away top Pfizer vaccine scientist
Bio Pharma Dive
NOVEMBER 30, 2021
Philip Dormitzer, who helped Pfizer develop shots for COVID-19, RSV and influenza, will become the British pharma's head of vaccine R&D next month.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
NOVEMBER 30, 2021
Philip Dormitzer, who helped Pfizer develop shots for COVID-19, RSV and influenza, will become the British pharma's head of vaccine R&D next month.
Bio Pharma Dive
JANUARY 23, 2023
Agency scientists are proposing to update COVID shots once a year to match circulating coronavirus strains, as well as simplifying current vaccination regimens.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
FEBRUARY 24, 2021
Agency scientists noted the shot's strong protection against severe COVID-19, even for the virus variant first detected in South Africa and known to weaken vaccine potency.
AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 12, 2024
That’s how many vaccines a man in Germany claims to have received for COVID-19 in just 29 months – and his body isn’t reacting the way some scientists thought it would. Two hundred and seventeen. The 62-year-old male from the city of Magdeburg made headlines a few years ago for his private and risky decision […]
Bio Pharma Dive
AUGUST 11, 2020
The shot, developed by the state-backed Gamaleya Research Institute, hasn't yet been tested in the large, placebo-controlled tests scientists emphasize are needed to prove a vaccine is protective.

Bio Pharma Dive
SEPTEMBER 9, 2022
David Kaslow, the lead scientist at a global public health nonprofit, will succeed Marion Gruber, who retired last fall amid her dissent on the timing of COVID-19 vaccine boosters.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 1, 2024
Scientists have developed a vaccine against a notorious drug-resistant superbug, targeting molecules on its surface that are also found on other bacteria and fungi.

Pharmaceutical Technology
AUGUST 17, 2022
On August 8, Pfizer and Valneva announced the initiation of a Phase III study with their Lyme disease vaccine , bringing the prospect of an injection to prevent the condition disease one step closer to reality. This means that the vaccine works against multiple serotypes of the disease, she explains. A one-size-fits-all approach.
AuroBlog - Aurous Healthcare Clinical Trials blog
APRIL 11, 2024
Using viruses that infect bacteria to detect proteins sprouted by a notorious parasite, scientists have honed in on possible vaccine targets for schistosomiasis, a neglected tropical disease that currently affects an estimated 600 million people worldwide, causing 280,000 deaths per year.

Bio Pharma Dive
DECEMBER 15, 2020
A review by agency staff affirmed the strongly positive results Moderna reported from a large Phase 3 study, clearing the way for a possible emergency authorization in the coming days.
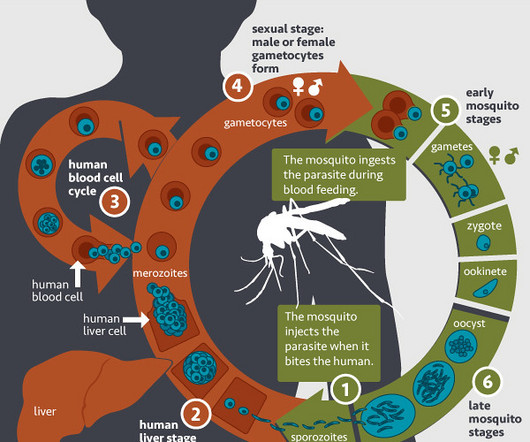
AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 8, 2023
The World Health Organization has approved a new vaccine that scientists argue will be a game-changer in the fight against malaria, which kills half a million people in Africa every year.

Bio Pharma Dive
DECEMBER 8, 2020
Agency staff didn't raise any red flags in their review of the companies' study results, documents released ahead of a key advisory committee meeting Thursday show.

Bio Pharma Dive
JULY 9, 2024
The company has begun searching for a successor to Dolsten, who oversaw more than 35 drug and vaccine approvals but also some notable setbacks during his time as Pfizer’s top scientist.

AuroBlog - Aurous Healthcare Clinical Trials blog
DECEMBER 14, 2022
The Department of Biotechnology (DBT) is extending support to the institutions and scientists to set up Centres of Excellence in the country for nano-vaccines and nano-adjuvants, to treat cancer and other diseases.The Department has invited Letters of Intent (LoIs) for ‘Setting up of CoE in Nano-Vaccines & Nano-Adjuvants’ for a period of five years (..)

Bio Pharma Dive
FEBRUARY 24, 2021
Moderna has delivered a modified version of its vaccine to NIH scientists, while Pfizer has begun testing of an additional booster shot in a clinical trial, both precautionary steps against emerging strains.

Bio Pharma Dive
NOVEMBER 11, 2021
The biotech claimed its NIH partners were involved only after a key discovery was made "by Moderna scientists using Moderna technology." " The dispute could end up in court.

NY Times
SEPTEMBER 13, 2020
Researchers say drug companies need to be more open about how vaccine trials are run to reassure Americans who are skittish about getting a coronavirus vaccine.

Bio Pharma Dive
FEBRUARY 24, 2021
The biotech has delivered a newly developed booster shot to NIH scientists, and will test several other approaches to address emerging virus strains.

pharmaphorum
JANUARY 8, 2021
The Pfizer/BioNTech vaccine appears to work against an important mutation in the new coronavirus variants causing havoc in the UK and South Africa, according to research from the drugs giant. The study team tested blood taken from people who had received the mRNA vaccine developed by BioNTech and Pfizer.

pharmaphorum
MAY 19, 2022
Moderna has joined forces with non-profit organisation IAVI on a third phase 1 trial of its candidate HIV vaccine in Africa, where the burden of the virus is still being keenly felt. There are hopes that its mRNA approach, which proved so effective against COVID-19, could succeed where traditional vaccine technologies have failed in HIV.

World of DTC Marketing
APRIL 11, 2021
QUICK THOUGHT: People believe the COVID-19 vaccine was developed in less than a year but that’s not true. Based on the virus a number of vaccines targeting the spike protein were designed, tested in animal models and found to be quite promising against SARS and other coronavirus illnesses like Middle East respiratory syndrome.

NY Times
NOVEMBER 10, 2020
The German company BioNTech, founded by two scientists, has teamed up with Pfizer on a vaccine that was found to be more than 90 percent effective.

pharmaphorum
JANUARY 18, 2021
Public health authorities in California are seeking a halt on dosing of one lot of Moderna’s COVID-19 vaccine after reports of allergic reactions at one immunisation clinic. The clinic in question switched to another lot of Moderna vaccine after closing for a few hours.

Pharma Times
DECEMBER 2, 2021
Scientists and health experts have cautioned that the new variant could have the ability to bypass the effectiveness of vaccines that are currently available.

BioSpace
NOVEMBER 10, 2021
?Scientists are conducting research into the possibility of creating a vaccine that activates T cells that targets not just SARS-CoV-2 but also its variations, even the ones that cause common colds.

Scienmag
JUNE 9, 2022
HAMILTON, June 10, 2022–McMaster University scientists who compared respiratory vaccine-delivery systems have confirmed that inhaled aerosol vaccines provide far better protection and stronger immunity than nasal sprays.

BioPharma Reporter
OCTOBER 3, 2023
The Nobel Prize in medicine has been awarded to two scientists who developed the technology which led to the mRNA vaccines against COVID-19.

pharmaphorum
DECEMBER 17, 2020
Tik Tok is unlikely to spring to mind as a source of reliable information about complex issues, but scientists are using it to fly the flag for COVID-19 vaccines and other health topics. When we talk about vaccines as health professionals, people who are vehemently anti-vaccine can take it out of context for their agenda.

pharmaphorum
MARCH 16, 2021
European regulators questioned the integrity of early batches of Pfizer/BioNTech’s mRNA vaccine, although the matter was resolved before approval, according to information leaked online following a cyberattack. As it conducted its analysis of the vaccine in December, the European Medicines Agency’s systems were targeted by unknown hackers.

NY Times
NOVEMBER 28, 2021
A “Frankenstein mix” of mutations raises concerns, but the variant may remain vulnerable to current vaccines. If not, revisions will be necessary.

pharmaphorum
JULY 22, 2024
Scientists at OHSU in the US have developed a universal form of influenza vaccine that they say could protect against all strains of the virus

Pharma Times
DECEMBER 6, 2021
With the emergence of the new Omicron variant bringing into question the effectiveness of current vaccines, scientists caution that viruses will continue to evolve.

STAT News
JANUARY 23, 2023
Scientists at the Food and Drug Administration propose making Covid vaccination a regular, once-a-year shot that is updated to match current strains of the SARS-CoV-2 virus, according to documents posted by the FDA on Monday. For people who are older or immunocompromised, the FDA would recommend two annual doses of the revised shot.

BioPharma Reporter
DECEMBER 23, 2020
People who have been infected with SARS-CoV-2 retain immune memory to protect against reinfection for 'at least eight months', according to Australian scientists. This research is the strongest evidence for the likelihood that vaccines against the virus will work for long periods," they say.

Medical Xpress
NOVEMBER 18, 2022
Rutgers scientists have developed a new approach to stopping viral infections: a so-called live-attenuated, replication-defective DNA virus vaccine that uses a compound known as centanamycin to generate an altered virus for vaccine development.
pharmaphorum
JANUARY 15, 2021
J&J has announced early trial results that suggest its single-shot coronavirus vaccine provides a sustained response against the virus ahead of a phase 3 trial readout due later this month. Both of these vaccines require two doses, as does the most recently approved shot from Moderna, which is due to arrive in the UK in the spring.

Bio Pharma Dive
OCTOBER 13, 2021
In briefing documents, agency scientists emphasized both vaccines still offer strong protection against severe COVID-19, but acknowledged potential for some added benefit with another dose.
Medical Xpress
NOVEMBER 24, 2022
An experimental mRNA-based vaccine against all 20 known subtypes of influenza virus has provided broad protection from otherwise lethal flu strains in initial tests, and thus might serve one day as a general preventative measure against future flu pandemics, according to researchers from the Perelman School of Medicine at the University of Pennsylvania. (..)

BioSpace
AUGUST 13, 2020
Phase III clinical trials for Russia’s Sputnik V vaccine for COVID-19 began Wednesday, one day after the Russia Direct Investment Fund (RDIF) launched a new website to share the details of the vaccine with the public and scientists around the world.
XTalks
JUNE 21, 2024
The US Food and Drug Administration (FDA) has green-lighted the first pneumonia vaccine specifically designed for adults 50 years of age and older. These numbers do not reflect the efficacies of the vaccines, which have not been compared head-to-head in any trial yet.

Medical Xpress
JANUARY 4, 2023
Scientists are harnessing a new way to turn cancer cells into potent, anti-cancer agents. The team tested their dual-action, cancer-killing vaccine in an advanced mouse model of the deadly brain cancer glioblastoma, with promising results. In the latest work from the lab of Khalid Shah, MS, Ph.D.,

Scienmag
OCTOBER 12, 2020
Credit: WEI Wei Exploration of new leukemia antigens and construction of appropriate delivery systems using FDA-approved material are important strategies for developing leukemia vaccines for clinic use.

STAT News
NOVEMBER 16, 2022
BOSTON — Even after leading the charge combating the worst pandemic in a century, vaccine researcher Kathrin Jansen doesn’t feel that she can relax. Another pandemic — this one based on an influenza virus — is inevitable, Jansen said at the annual STAT Summit on Tuesday. Read the rest…

World of DTC Marketing
NOVEMBER 19, 2021
government has provided Moderna with nearly $10 billion in taxpayer money for research and development and the purchase of 500 million doses of this mRNA COVID-19 vaccine. government made available to them to make this COVID-19 vaccine. billion for the development of its vaccine and to pay for doses once approved.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content