CanSino, Canada abandon plans for a coronavirus vaccine trial
Bio Pharma Dive
AUGUST 27, 2020
Canada was set to help CanSino produce its experimental shot as part of a deal to run what would've been the country's first coronavirus vaccine study.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
AUGUST 27, 2020
Canada was set to help CanSino produce its experimental shot as part of a deal to run what would've been the country's first coronavirus vaccine study.

Bio Pharma Dive
SEPTEMBER 17, 2020
The biotech, among the furthest along in coronavirus vaccine development, is the first developer to share the so-called study protocol of its large Phase 3 trial.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Bio Pharma Dive
SEPTEMBER 15, 2020
Importantly, the study committee hasn't had reason to pause vaccinations in the trial, something AstraZeneca and the University of Oxford recently reported in tests of their vaccine.

Bio Pharma Dive
NOVEMBER 11, 2024
After an investigation, the agency concluded that a case of muscle weakness in one of Novavax’s trials was actually ALS and unrelated to vaccination.

Bio Pharma Dive
JANUARY 25, 2021
The drugmaker, one of the largest vaccine developers globally, won't move forward with either of two candidates it's been testing after disappointing data in early trials.

World of DTC Marketing
DECEMBER 29, 2020
OBSERVATION: Biologics can take a long time to develop but COVID vaccines have been in development for almost 50 years and novel approaches were used to develop these vaccines. Vaccines typically take 10 to 15 years to develop, test and release to the public. The coronavirus vaccines, however, took less than a year.

Bio Pharma Dive
JANUARY 29, 2021
The vaccine's efficacy was higher in the U.S., but lower in Latin America and South Africa, where new, more infectious virus variants are circulating.

Bio Pharma Dive
FEBRUARY 10, 2023
The pharma and partner BioNTech have kicked off the first human trial of a messenger RNA shot for shingles, believing there’s room to clear the high bar set by GSK’s rival vaccine.

Bio Pharma Dive
JUNE 14, 2021
Highly anticipated results from a Phase 3 study testing the biotech company's shot showed it to be strongly protective and safe, a potential boon for the world's vaccination efforts.
Pharmaceutical Technology
MAY 11, 2023
Results published in Nature for a personalised pancreatic cancer vaccine that uses neoantigens from patients’ tumours have lent further support to early positive signals. The vaccine, developed by BioNTech, led to half of the patients with pancreatic cancer in the Phase I trial remaining cancer-free 18 months later.

Pharmaceutical Technology
NOVEMBER 12, 2024
The FDA agreed for Novavax to continue trials of its vaccine combo after addressing a serious adverse event that paused trials.

Bio Pharma Dive
SEPTEMBER 22, 2021
Positive results from Clover's Phase 3 study could mean another coronavirus vaccine option for the many countries still grappling with short supplies.

Bio Pharma Dive
SEPTEMBER 8, 2020
A participant in a clinical trial of the experimental shot suffered from an unexplained illness, triggering an investigation and a pause in vaccinations.

Bio Pharma Dive
JANUARY 26, 2022
New data, meanwhile, help affirm the benefit of a third dose of Moderna's current vaccine. The study will test a version of the biotech's COVID-19 shot that's tailored to the infectious variant.

Bio Pharma Dive
MARCH 8, 2021
Results from previous trials have raised questions about the vaccine and how well it works. Data from a large U.S. study could help clear up the confusion.

Bio Pharma Dive
SEPTEMBER 15, 2020
The Serum Institute of India, a go-to partner for coronavirus vaccine makers, will help Novavax boost capacity to levels matching its larger rivals.

Pharmaceutical Technology
MARCH 26, 2025
Sanofi will commence a Phase I/II trial with its vaccine candidate in the next few days to start generating immunogenicity data.

Pharmaceutical Technology
MARCH 28, 2023
Following on from its Covid-19 vaccine programmes, BioNTech has set its sights on a range of infectious diseases for vaccine development. The company saw major successes with its Covid-19 vaccine, developed in collaboration with Pfizer. In response to the lower vaccine sales forecasts, BioNTech shares opened at 3.9%
Pharmaceutical Technology
FEBRUARY 3, 2023
Only a few weeks into the new year, the prospect of getting a successful advanced HIV vaccine shrank after the discontinuation of yet another late-stage trial. On January 18, Janssen, a Johnson & Johnson (J&J) subsidiary, stated that its vaccine was not effective in preventing HIV infections.

Bio Pharma Dive
NOVEMBER 12, 2020
has sped up Moderna's timeline for its late-stage vaccine study, which now has sufficient COVID-19 cases for a first look at results. Coronavirus' fast accelerating spread in the U.S.

Bio Pharma Dive
AUGUST 11, 2020
has already invested heavily in Moderna's vaccine, having previously committed $1 billion to fund clinical trials and manufacturing scale-up.

Pharmaceutical Technology
JUNE 16, 2023
France-based biotech company Osivax has thrown its hat into the influenza ring by dosing the first subject with its vaccine candidate OVX836. The Phase IIa trial (NCT05734040) in Australia will see a potential 500 volunteers given OVX836 in combination with quadrivalent influenza vaccines (QIVs).

Pharmaceutical Technology
MARCH 14, 2023
The National Health Surveillance Agency (ANVISA) in Brazil has granted approval for Takeda ’s dengue virus vaccine candidate, Qdenga (Dengue Tetravalent Vaccine [Live, Attenuated]) (TAK-003). The vaccine has received approval for preventing dengue disease in people aged four years to 60 years.

Bio Pharma Dive
OCTOBER 20, 2020
The co-leader of an NIH network of coronavirus prevention studies spoke with BioPharma Dive about the FDA's big vaccine meeting this week and what will come next.

Bio Pharma Dive
NOVEMBER 17, 2020
Positive results from Pfizer and Moderna offer the strongest validation yet of researchers' approach to coronavirus vaccines, but critical questions remain.
Bio Pharma Dive
OCTOBER 25, 2021
The trial results should allow the company to ask the FDA for approval of its vaccine in younger children, just as Pfizer nears a crucial decision for its shot in 5- to 11-year olds.
Pharmaceutical Technology
FEBRUARY 7, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorisation for Takeda ’s dengue virus vaccine candidate, Qdenga (Dengue Tetravalent Vaccine [Live, Attenuated]). The vaccine candidate has been approved for active immunisation against the infection in people from four years of age.

Pharmaceutical Technology
APRIL 14, 2023
Ghana’s Food and Drug Authority (FDA) has approved R21/Matrix-M malaria vaccine in children aged 5 to 36 months, marking the first regulatory clearance for the University of Oxford-developed vaccine in any country in the world. Children between the ages of five and 36 months are at highest risk of death from malaria.
Pharmaceutical Technology
SEPTEMBER 7, 2022
Indian company Bharat Biotech International has secured approval from Central Drugs Standard Control Organisation (CDSCO) under Restricted Use in Emergency Situation for its intranasal Covid-19 vaccine, iNCOVACC (BBV154), for usage in people aged 18 years and older. It is formulated to permit intranasal delivery via nasal drops.
Pharmaceutical Technology
DECEMBER 7, 2022
Health Canada has granted approval for a supplement to a New Drug Submission (sNDS) of Novavax ’s Covid-19 vaccine (Recombinant protein, Adjuvanted), Nuvaxovid (NVX-CoV2373), for use in adolescents aged 12 to 17 years. The trial is designed to analyse the safety and effectiveness of the vaccine.

Bio Pharma Dive
SEPTEMBER 28, 2020
The FDA has suspended the planned start of a late-stage trial of Inovio's experimental shot, dealing another setback to one of the most advanced DNA-based vaccine programs in development.

Pharmaceutical Technology
AUGUST 17, 2022
On August 8, Pfizer and Valneva announced the initiation of a Phase III study with their Lyme disease vaccine , bringing the prospect of an injection to prevent the condition disease one step closer to reality. This means that the vaccine works against multiple serotypes of the disease, she explains. In June 2022, Pfizer acquired 8.1%

Pharmaceutical Technology
JUNE 24, 2022
Novavax has obtained emergency use authorization (EUA) for its Covid-19 vaccine, Nuvaxovid (NVX-CoV2373), from the Taiwan Food and Drug Administration for use in people of the age 18 years and above. The protein-based vaccine is engineered from the genetic sequence of the SARS-CoV-2 virus’ initial strain.

Pharmaceutical Technology
SEPTEMBER 5, 2024
Moderna is currently running a Phase I/II trial of the mRNA mpox vaccine mRNA-1769 in healthy participants.
Pharmaceutical Technology
OCTOBER 14, 2022
German pharmaceutical firm Merck has extended its partnership with Moderna to jointly develop and sell mRNA-4157/V940, an investigational personalised cancer vaccine (PCV). They subsequently expanded the collaboration to include the development of antigen mRNA cancer vaccines in 2018.
AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 3, 2023
An experimental vaccine that aims to slow down or prevent the progression of Alzheimer’s disease has been trialed in mice with promising early results. Mice engineered with genes that put them at greater risk of an Alzheimer’s-like disease had fewer amyloid plaques following the vaccine.

Bio Pharma Dive
NOVEMBER 18, 2020
Reaching the main goal of their large trial, Pfizer and BioNTech reported 162 cases of COVID-19 among participants who received a placebo, and just 8 for those who were vaccinated.

Pharmaceutical Technology
JUNE 9, 2023
5+delta) protein vaccine (Sf9 cell). This marks the world’s first Covid-19 vaccine approved for emergency use against XBB descendent lineages of SARS-CoV-2. The vaccine has been developed by WestVac Biopharma along with the West China Medical Center and Sichuan University. recombinant Covid-19 trivalent (XBB.1.5+BA.5+delta)

Bio Pharma Dive
FEBRUARY 4, 2021
The government-funded trial will evaluate the effects of mixing vaccines and varying timing between shots.

Bio Pharma Dive
DECEMBER 10, 2021
Data from a small Phase 1 trial showed the biotech's vaccine spurred immune responses to four common strains, although they didn't appear significantly greater than an already available shot from Sanofi.

Bio Pharma Dive
OCTOBER 16, 2024
The hold, which was made in response to a serious adverse event report, could impact the company’s plans to start a Phase 3 trial of a combination shot for COVID-19 and influenza.
Pharmaceutical Technology
SEPTEMBER 5, 2022
The National Medical Products Administration of China (NMPA) has approved CanSino Biologics ’ (CanSinoBIO) recombinant Covid-19 vaccine (Adenovirus Type 5 Vector) for inhalation, Convidecia Air, as a booster. This vaccine leverages the same adenovirus vector technological platform as Convidecia, the intramuscular version.
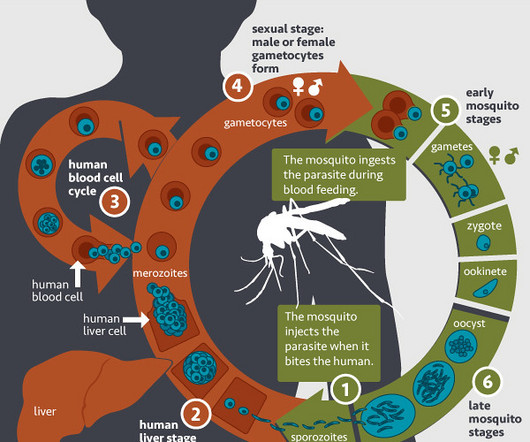
AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 8, 2023
The World Health Organization has approved a new vaccine that scientists argue will be a game-changer in the fight against malaria, which kills half a million people in Africa every year.
Pharmaceutical Technology
JULY 15, 2022
Health Canada has granted approval for the usage of Moderna’s messenger RNA (mRNA) Covid-19 vaccine, Spikevax, in a 25µg two-dose regimen for active immunisation to prevent Covid-19 in children aged six months to five years. So far, children aged below five years were not eligible to receive the Covid-19 vaccine in Canada.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content